Analyses of the crystal packing of (Ph3P)2Ag(O2C2O2)Ag(PPh3)2 and related heavily phenylated molecules: substitutional trimorphism
Philip A. W.
Dean
*a,
Marcia
Scudder
b,
Don
Craig
b and
Ian
Dance
*b
aDepartment of Chemistry, University of Western Ontario, London, Ontario, Canada N6A 5B7. E-mail: pawdean@uwo.ca
bSchool of Chemistry, University of New South Wales, Sydney, NSW 2052, Australia. E-mail: i.dance@unsw.edu.au
Abstract
We describe the crystallisation and crystal structure of (Ph3P)2Ag(O2C2O2)Ag(PPh3)2, and analyse its crystal packing and crystal supramolecularity in the context of a set of six related molecules of the type (Ph3P)2M(X1X3C2X2X4)M(PPh3)2, M⊕=⊕Cu, Ag; Xi⊕=⊕O, S. These molecules are heavily phenylated, and intramolecular and intermolecular motifs involving mainly edge-to-face (EF) phenyl rings are dominant. There are three different crystal packings (A, B, C) representing substitutional trimorphism, because the internal substitution does not necessarily influence crystal packing. There are intramolecular EF interactions between different Ph3P ligands at the end and across the bridge of the complexes, and these intramolecular EF pairs mesh to generate many additional intermolecular EF motifs. The dominant multiple phenyl embraces are (EF)4, (EF)∞ ribbons, and (EF)2(OFF) (OFF⊕=⊕offset-face-to-face), with one instance of triangular (EF)3. The sixfold phenyl embrace (6PE) comprised of (EF)6 occurs only in trimorph A, adopted by (Ph3P)2Ag(O2C2O2)Ag(PPh3)2. Intramolecular and intermolecular C–H⋯X hydrogen bonding occurs, but is not a major influence. The mutual interactions of the intramolecular stereochemistry of the complexes and the crystal packing are discussed.
Introduction
We are interested in the crystal packing of molecules that have a high proportion of phenyl groups over their surfaces, and therefore have the potential to engage in multiple phenyl embraces (MPE). This is part of an investigation of the variety and the strengths of multiple phenyl embraces as supramolecular motifs, and therefore of their value in crystal design and engineering. One variable for these molecules is the intramolecular conformational flexibility of the phenyl groups, and the extent to which they can flex to permit formation of MPE. In this context there are many molecules where the phenyl groups belong to PPh3 ligands (or derivatives) and are partly constrained.1 We have analysed the intramolecular and supramolecular stereochemistry of M–PPh3 complexes,1,2 and documented some high-order MPE and high symmetry lattices,3 the non-interference by substituents on the phenyl groups,4 and the formation of a diamondoid lattice maintained only by MPE in [Cu{P(C6H4-4-OCH3)3}3]+ ClO4−.5 Here, we describe the crystallisation and crystal structure of (Ph3P)2Ag(O2C2O2)Ag(PPh3)2, analyse its crystal packing and its crystal supramolecularity in relation to that of similar molecules in which a tight, inflexible, oxalate-derived bridge, 1, links two (Ph3P)2M moieties.The set of compounds considered involves five from the Cambridge structural database in addition to (Ph3P)2Ag(O2C2O2)Ag(PPh3)2
(see Table 1). There are three different crystal lattice (packing) types: one (A) in P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (including the new compound reported in this paper), and two different lattices (B and C) in space group P21/c. It can be noted from Table 1 that there is no correlation of the molecular composition (M, X) with the lattice packing type, and accordingly this set of six compounds can be considered to demonstrate three substitutional polymorphs. In conventional definitions, polymorphs are crystal packing isomers of the one compound or molecule.6–11
Substitutional polymorphism extends the definition to include molecules related by homologous atomic substitution, as is commonly pertinent in inorganic, coordination and organometallic contexts.12,13
(including the new compound reported in this paper), and two different lattices (B and C) in space group P21/c. It can be noted from Table 1 that there is no correlation of the molecular composition (M, X) with the lattice packing type, and accordingly this set of six compounds can be considered to demonstrate three substitutional polymorphs. In conventional definitions, polymorphs are crystal packing isomers of the one compound or molecule.6–11
Substitutional polymorphism extends the definition to include molecules related by homologous atomic substitution, as is commonly pertinent in inorganic, coordination and organometallic contexts.12,13
| CSD reference code | M | X1 | X2 | X3 | X4 | Space group | Approximate cell dimensions | Crystal packing class | |
|---|---|---|---|---|---|---|---|---|---|
| a, b, c/Å | (α), β, (γ)/° | ||||||||
| a (Ph3P)2Cu(S2C2SO)Cu(PPh3)2 has disordered S/O sites. | |||||||||
| This work | Ag | O | O | O | O |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
9.9, 13.2, 13.4 | 64, 79, 88 | A |
| DETZIL14 | Cu | S | S | S | S |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
10.2, 13.1, 13.5 | 63, 89, 78 | A |
| TENFUNa15 | Cu | S | S/O | S/O | S |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
10.2, 13.1, 13.5 | 116, 90, 101 | A |
| CEFMIJ16 | Ag | S | O | O | S | P21/c | 13.2, 11.9, 20.9 | 101.4 | B |
| YOFMUB17 | Cu | S | S | O | O | P21/c | 23.6, 13.7, 20.5 | 104.1 | C |
| YOFMUF17(no coordinates) | Ag | S | S | O | O | P21/c | 23.6, 13.7, 20.5 | 103.9 | C |
Results
Finely-divided silver oxalate (0.110 g, 0.362 mmol) was allowed to stand in contact with a solution of triphenylphosphine (0.319 g, 1.22 mmol) in acetone (4 mL) for 2 days at room temperature. The white product was separated by filtration and dried. Isolated yield 0.258 g (0.19 mmol); 63%. A portion was recrystallised from dimethylformamide/acetonitrile to give crystals suitable for X-ray analysis.Reflection data were measured using an Enraf-Nonius CAD-4 diffractometer in θ/2θ scan mode using graphite monochromated molybdenum radiation (λ⊕=⊕0.7107 Å). The structure was determined by direct phasing and Fourier methods. The asymmetric unit consists of half of one molecule. The Ag, P, O and oxalate C atoms were refined anisotropically. A single ring was used to model all six phenyl rings with their location and orientation as variables. Hydrogen atoms were included in calculated positions. The thermal motion of each phenyl ring was described by a 12 parameter TL model (where T is the translation tensor, L is the libration tensor). Crystal data and details of refinement are given in Table 2.
| Parameter | |
|---|---|
| a Click b160322.txt for full crystallographic data (CCDC 160322). | |
| Empirical formula | C74H60Ag2O4P4 |
| M | 1352.9 |
| Crystal system | Triclinic |
| Space group |
P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) |
| a/Å | 9.923(6) |
| b/Å | 13.176(7) |
| c/Å | 13.364(7) |
| α/° | 63.57(4) |
| β/° | 78.61(4) |
| γ/° | 88.11(3) |
| V/Å3 | 1531(2) |
| Z | 1 |
| T/K | 294 |
| D c/g cm−3 | 1.47 |
| μ(MoKα)/mm−1 | 0.79 |
| 2θmax/° | 44 |
| Crystal decay (%) | 18 |
| Min transmission factor | 0.93 |
| Max transmission factor | 0.96 |
| Unique reflections | 3992 |
| Observed reflections | 3038 |
| R merge | 0.016 |
| R | 0.032 |
| R w | 0.038 |
Crystal structure and packing of (Ph3P)2Ag(O2C2O2)Ag(PPh3)2
The molecule is centrosymmetric, with tetrahedral stereochemistry at Ag; bond lengths are normal. There are significant intramolecular Ph⋯Ph interactions between different Ph3P ligands, illustrated in Fig. 1. All of the intramolecular Ph⋯Ph interactions are edge-to-face, EF. At each end of the molecule there is an (EF)2 motif between the two crystallographically independent Ph3P ligands, and across the centre of the molecule, covering the oxalate, there are two more EF motifs. All Ph rings are engaged in an EF interaction with a Ph ring on a different ligand. As will become evident, these EF and (EF)2 motifs within the molecule are all involved in intermolecular motifs that are key components of the crystal packing. | ||
| Fig. 1 Centrosymmetric (Ph3P)2Ag(O2C2O2)Ag(PPh3)2 molecule (Ag black, P magenta, O red). There are two edge-to-face (EF) Ph⋯Ph interactions between the two ends of the molecule, and at each end there are two EF motifs between the two ligands. Note that all Ph rings are engaged in an EF with a ring on a different Ph3P ligand. | ||
There are intramolecular and intermolecular C–H⋯O hydrogen bonds: those within the molecule are shown in Fig. 2, which also provides the atom labelling used. Only O1 is involved in intramolecular C–H⋯O hydrogen bonding.
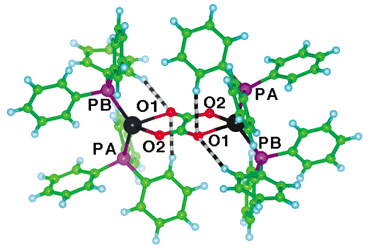 | ||
| Fig. 2 Well developed C–H⋯O hydrogen bonds (H⋯O⊕=⊕2.54, 2.55 Å) within (Ph3P)2Ag(O2C2O2)Ag(PPh3)2, involving only O1. Click image or 2.htm to access a 3D representation. | ||
The overall crystal packing is shown in Fig. 3. The intermolecular hydrogen bonds link the molecules in layers parallel to ab, centred at z⊕=⊕0.5. One type of sixfold phenyl embrace (6PE) occurs between the Ph3P at PB, connecting the layers of molecules that are linked by hydrogen bonds. The 6PE is centrosymmetric [at centres of inversion of type (1/2, 1/2, 0 – site e)], with a P⋯P distance of 6.94 Å.
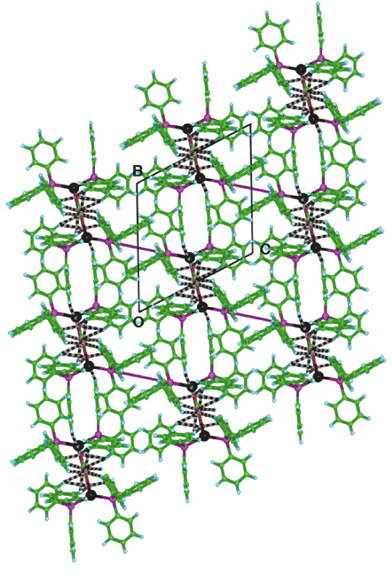 | ||
| Fig. 3 Overall packing of (Ph3P)2Ag(O2C2O2)Ag(PPh3)2 molecules and the hydrogen bonds (black and white candystripes) and 6PE (magenta rods) which link them, projected along the a axis. The intermolecular C–H⋯O bonds occur only in layers parallel to the ab plane, and the 6PE which occur at only one end of each molecule provide diagonal linkages between the layers. | ||
Further details of the intermolecular hydrogen bonds within the layer are presented in Fig. 4. Also evident in Fig. 4 is another type of intermolecular motif, in which the intramolecular EF pairs of phenyl rings mesh to form centrosymmetric intermolecular (EF)2 pairs: this meshing of two pairs of phenyl rings generates a total of four EF interactions (two intramolecular, two intermolecular) and these motifs are labelled (EF)4 in the following. One set of these occurs laterally between molecules, approximately in the plane of the oxalate bridge, and another set occurs between molecules end-to-end. One example of each type is marked on Fig. 4. These two (EF)4 motifs occur within the ab layer of molecules, and straddle centres of inversion of type (1/2, 0, 1/2) for the lateral motif and (0, 1/2, 1/2) for the end motif. The lateral motif occurs as isolated (EF)4, but the end motif forms a continuous ribbon (EF)∞ through the crystal in the a direction.
 | ||
| Fig. 4 Independent intermolecular C–H⋯O hydrogen bonds within the ab layer of molecules in (Ph3P)2Ag(O2C2O2)Ag(PPh3)2. The H⋯O distances for the intermolecular bonds are 2.88 and 2.96 Å at O1, and 2.75, 2.82, 2.99 and 3.10 Å at O2. The upper rectangle surrounds the phenyl rings of the lateral (EF)4 intermolecular motif, and the lower rectangle draws attention to part of the ribbon of EF interactions involving the ends of molecules. | ||
A detail of each motif is provided in Fig. 5. The lateral motif [Fig. 5(a)] has a total of four EF interactions: two intramolecular (but inter-Ph3P ligand) and two intermolecular. There are additional EF interactions in the end, ribbon, motif [Fig. 5(b)]. The full array and the high density of these (EF)4 motifs within the hydrogen bonded layer of molecules is evident in the space-filling representation of Fig. 6.
 | ||
| Fig. 5 Detail of the intermolecular (EF)n motifs occurring within the ab layers of hydrogen-bound molecules in crystalline (Ph3P)2Ag(O2C2O2)Ag(PPh3)2. (a) Four phenyl rings of the lateral (EF)4 around centres of inversion of type (1/2, 0, 1/2 – site f). (b) Part of the ribbon of EF interactions for phenyl rings at the ends of the molecules, around centres of inversion of type (0, 1/2, 1/2 – site g) and (1/2, 1/2, 1/2 – site h). | ||
 | ||
| Fig. 6 Close packing of the full array of intermolecular and intramolecular (EF)2 motifs involving (Ph3P)2Ag(O2C2O2)Ag(PPh3)2 molecules within the hydrogen bonded ab layer. The lateral (EF)4 motifs are central at the top and bottom, whereas the continuous (EF)∞ ribbon is horizontal in the centre of the figure. The orientation of this figure is similar to that of Fig. 4. | ||
Finally, there is another well developed multiple phenyl embrace occurring between the layers of hydrogen bonded molecules around centres of inversion of type (0, 0, 0). As shown in Fig. 7, this embrace occurs laterally between two molecules, but is approximately perpendicular to the bridging oxalate ligand, in contrast to the lateral (EF)4 motif already shown in Fig. 4–6. The embrace uses three phenyls per molecule, two of which are an intramolecular EF pair. These mesh between molecules to form two new intermolecular EF motifs and a central offset-face-to-face (OFF) motif. The (EF)2(OFF) intermolecular component of this is like that of the P4PE,18 which uses only four phenyl rings.
 | ||
| Fig. 7 Phenyl embrace between two molecules across the centre of inversion of type (0, 0, 0). There are two intermolecular EF motifs, two intramolecular EF motifs, and one offset-face-to-face (OFF) motif in the centre: in the OFF there is a close C⋯C distance of 3.37 Å. | ||
Conclusions about the crystal supramolecularity of (Ph3P)2Ag(O2C2O2)Ag(PPh3)2
These crystals of (Ph3P)2Ag(O2C2O2)Ag(PPh3)2 are efficiently packed. Multiple phenyl embraces comprised of EF local motifs are dominant through the crystal lattice, and are supported by some C–H⋯O bonds. The intermolecular meshing of intramolecular EF motifs is a distinctive feature, and of the better known multiple phenyl embraces usually occurring for complexes with Ph3P ligands,1,19 only the 6PE occurs in this crystal. It would appear, based on the relative numbers and the dimensions of the motifs, that the multiple phenyl embraces provide the greater influence. This is consistent with the energies (calculated) of C–H⋯O bonds (<4 kcal mol−1)20 and phenyl⋯phenyl interactions (ca. 2 kcal mol−1 per Ph2, ca. 10 kcal mol−1 per 6PE).1,19Alternative crystal packing, type B
The analogous compound with the trans-dithiooxalate bridging ligand, (Ph3P)2Ag(OSC2OS)Ag(PPh3)2 (CEFMIJ in Table 1), has a different crystal lattice.21 The centrosymmetric intramolecular conformation is similar to that of (Ph3P)2Ag(O2C2O2)Ag(PPh3)2 [see Fig. 8(a)] with (EF)2 interactions between ligands at the ends of the molecule, and (poorer) EF interactions between the ends. The intramolecular C–H⋯S has a H⋯S distance of 3.1 Å. | ||
| Fig. 8 Intramolecular conformations of molecules in crystal lattices of type B (a) and of type C (b) (S yellow). | ||
Fig. 9 shows the crystal packing of CEFMIJ, and it is evident that there is intermolecular meshing of EF motifs, and similarities with Fig. 4 and Fig. 6 for (Ph3P)2Ag(O2C2O2)Ag(PPh3)2.
 | ||
| Fig. 9 Crystal packing of CEFMIJ, highlighting the region of intermeshed EF interactions. Ribbons of EF interactions parallel to b are generated by centres of inversion at (1, 1/2, 1/2 – type a) and (1, 0, 1/2 – type c). | ||
Fig. 10 shows two intermolecular embraces, based on EF motifs, occurring in CEFMIJ. There is an (EF)2(OFF) lateral embrace of molecules, shown in Fig. 10(a), which has some similarities with the analogous embrace of Fig. 7. Further, an EF pair of phenyl rings at the ends of the molecules intermesh to form a continuous ribbon of EF motifs, shown in Fig. 10(b). This embrace is broadly related to the ribbon embrace of (Ph3P)2Ag(O2C2O2)Ag(PPh3)2 (see Fig. 5), but there are also clear differences. The 6PE that occurs in (Ph3P)2Ag(O2C2O2)Ag(PPh3)2 is absent from CEFMIJ.
![Two EF-based embraces between (Ph3P)2Ag(OSC2OS)Ag(PPh3)2 molecules in CEFMIJ. (a) Lateral (EF)2(OFF) embrace around centres of inversion of type b (1/2, 1/2, 1/2) linking molecules laterally along the b direction [cf.Fig. 7 for (Ph3P)2Ag(O2C2O2)Ag(PPh3)2]. (b) Continuous ribbon of EF motifs, propagated by centres of inversion at (0, 0, 1/2) and (0, 1/2, 1/2), linking the ends of molecules.](/image/article/2001/CE/b102399n/b102399n-f10.gif) | ||
| Fig. 10 Two EF-based embraces between (Ph3P)2Ag(OSC2OS)Ag(PPh3)2 molecules in CEFMIJ. (a) Lateral (EF)2(OFF) embrace around centres of inversion of type b (1/2, 1/2, 1/2) linking molecules laterally along the b direction [cf.Fig. 7 for (Ph3P)2Ag(O2C2O2)Ag(PPh3)2]. (b) Continuous ribbon of EF motifs, propagated by centres of inversion at (0, 0, 1/2) and (0, 1/2, 1/2), linking the ends of molecules. | ||
Both the motifs shown above occur within layers in the ab plane (Fig. 11). Adjacent layers are generated by the glide and screw symmetry operators, and there are no good Ph⋯Ph interactions between the layers.
 | ||
| Fig. 11 Layer in CEFMIJ within which significant interactions occur. The (EF)2(OFF) can be seen at (1/2, 1/2) while the (EF)∞ ribbon is located at x⊕=⊕1 and is parallel to b. | ||
Alternative crystal packing, type C
The related molecule with Cu in place of Ag, and with cis-dithiooxalate as the bridge (YOFMUB in Table 1), has a different crystal packing type. The molecule cannot be centrosymmetric, and the development of intramolecular EF motifs [see Fig. 8(b)] is different and poorer than in the two preceding structures.The structure can be described as containing layers, which follow the ac diagonal of the cell (Fig. 12).
 | ||
| Fig. 12 Crystal packing of YOFMUB (Cu blue): the layer structure is evident. Note the absence of the intermeshed (EF) rings which were evident in Fig. 4 and 9 for structure types A and B. | ||
Within the layers there is little in the way of conventional phenyl⋯phenyl interactions. However, there is a composite arrangement where phenyl rings from two PPh3 groups on one Cu atom interact with those from a single PPh3 on a 21-related Cu atom. There is a total of seven phenyl rings involved in this ‘7-embrace’ (Fig. 13). Similar configurations are found at each of the two independent Cu atoms.
 | ||
| Fig. 13 Layer of (Ph3P)2Cu(SOC2OS)Cu(PPh3)2 found in YOFMUB. On the left of the figure there is a zig-zag of Cu2⋯Cu2 interactions, whereas on the right there is a similar zig-zag of Cu1⋯Cu1 interactions, both generated by the 21 axes (vertical in this view). The 7-embrace occurs at both interaction regions. | ||
The 7-embrace for Cu2⋯Cu2 is shown in detail in Fig. 14. There are two distinct surfaces to the ‘embrace’. On one side there is a triangle of rings forming (EF)3, whereas on the other surface there are four phenyl rings engaged in a pair of EF interactions, along with a single intramolecular EF.
 | ||
| Fig. 14 Two opposite views of the 7-embrace between Cu2 atoms in YOFMUB: the rings involved on each side are coloured orange. (Left) the triangular (EF)3, (right) the (EF)2 between four phenyl rings. | ||
Between the layers there is a centrosymmetric (EF)2 at (1/2, 1/2, 1/2) (see Fig. 15). This is similar to the (EF)2(OFF) motifs found in both structure types A and B, but here the OFF component is missing because the two parallel rings are laterally displaced and do not overlap.
 | ||
| Fig. 15 Centrosymmetric (EF)2 motif at (1/2, 1/2, 1/2). In contrast to the motif found in A- and B-type packing, the OFF component is missing because the two rings have slipped laterally and no longer overlap. However, the overall features of this ‘embrace’ persist. | ||
Intramolecular and intermolecular C–H⋯O hydrogen bonds are well developed in this structure.
Discussion
This paper describes three different crystal structures adopted by six very similar molecules. We regard these as substitutional trimorphs,12,13 because the difference between the molecules is brought about by homologous substitutions (Cu/Ag; O/S) at internal positions, not the periphery. The conventional view of polymorphism is of different crystal packing arrangements for the same molecule, and this is suitable for the mainly organic species to which it is applied. However, as illustrated nicely by the current set of coordination compounds, substitution within the same group of the periodic table yields chemically different species which need not have different supramolecular interactions, and so manifest polymorphism when they do have different crystal packing.The validity of this view is evident in Table 1: YOFMUB and YOFMUF have Cu/Ag substitution but the same crystal structure, indicating that metal substitution need not affect the packing; TENFUN has O/S disorder, indicating that O/S substitution need not affect the packing; the pairs TENFUN/YOFMUB and YOFMUF/CEFMIJ have these same substitutions and yet have different crystal packing arrangements. There is a variability of crystal packing that is not necessarily caused by the substitution, and therefore there is polymorphism.
There is some variability of molecular dimensions and conformation, as illustrated in Fig. 16. The similarity of the phenyl ring array for A and B, but the difference for C, is evident. In addition, there is variation of the orientation of the bridging oxalate ligand relative to the M2P4 plane of coordination, and here B is different from A and C. This affects details of the pseudo-tetrahedral coordination stereochemistry at the metals. The M⋯M distances for A, B and C are approximately 6.1, 6.5 and 5.7 Å, respectively.
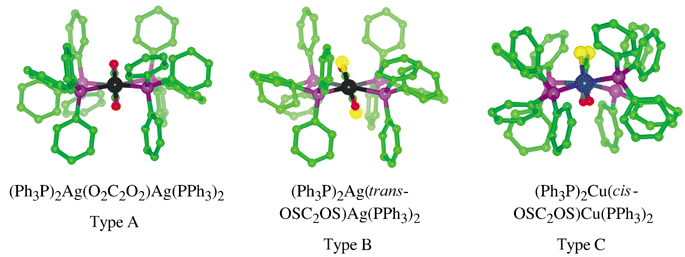 | ||
| Fig. 16 Comparison of the molecular structures for the trimorphs A, B and C, viewed along the M⋯M vector. Note the similarities and differences in the arrays of phenyl rings, and the orientation of the (thio)oxalato ligand. | ||
All three molecules display extensive intramolecular interaction between the phenyl rings of different Ph3P ligands which cover them. However, the interactions observed for type A are better developed than those for type B, which are again better than those for type C (see Fig. 16). It is reasonable to surmise that that interactions for A are better because the M(oxalate)M core has the optimum size.
A key question for crystal packing and polymorphism of non-rigid molecules is the mutual influence of variations in intramolecular stereochemistry and variations in intermolecular interactions.22 The differences in the three molecular structures (Fig. 16) are accompanied by differences in their crystal packing. However, there is a recurrence of packing motifs. One, the (OFF)(EF)2, occurs with variation in all three structures, being formed around a centre of inversion and linking the ends of two molecules. The phenyl rings involved are from both PPh3 groups on one metal atom, with one group contributing two rings, and the other, one. The motif is modified in C, because the OFF interaction has been eliminated by lateral displacement of the parallel rings which might form it. This is analogous to variations observed for the P4AE [(OFF)(EF)2] interaction of a pair of M(phen)2 moieties,23 where reorientation of the interacting rings can lead to the elimination of the OFF interaction, leaving only (EF)2. Another recurring motif is the intermolecular meshing of intramolecular (EF)2 regions. This can lead to isolated (EF)4 regions, or can result in infinite ribbons (EF)∞. Both of these motifs occur in trimorph A, but only the second in trimorph B, and neither occurs in trimorph C, which instead contains the 7-embrace. The occurrence of intermeshed EF interactions in A and B is enhanced by their centrosymmetric molecular symmetry. The good intramolecular EF interactions at the ends of the molecules are (necessarily) reproduced at the other ends, and then inversion centres create the (EF)4 [or (EF)∞] motifs. The constitutional asymmetry and skewing of molecular C would make such an alignment of rings more difficult.
Of the three structures, (Ph3P)2Ag(O2C2O2)Ag(PPh3)2 has the most regular intramolecular stereochemistry, and apparently the best crystal packing. The familiar multiple phenyl embrace for Ph3X systems, the 6PE which is (EF)6, occurs only once in these three crystal structures (in A), and the structures appear to be dominated by the alternative (EF)n multiple phenyl embraces described.
All three structures incorporate C–H⋯X (X⊕=⊕O and/or S) hydrogen bonding, both intramolecular and intermolecular. However we believe, based on the relative numbers and the dimensions of the motifs, that the multiple phenyl embraces provide the greater influence. This is consistent with the energies (calculated) of C–H⋯O bonds (<4 kcal mol−1)20 and of phenyl⋯phenyl interactions (ca. 2 kcal mol−1 per Ph2, ca. 10 kcal mol−1 per 6PE).1,19 The lack of influence of substitution of X on the crystal supramolecularity implies that C–H⋯O and C–H⋯S hydrogen bonding types are unimportant, or similar.
Acknowledgements
P. A. W. D. is grateful to the University of Western Ontario for a leave of absence during which this work was carried out at UNSW. This research is supported by the Australian Research Council.References
- I. G. Dance and M. L. Scudder, J. Chem. Soc., Dalton Trans., 2000, 1579 RSC.
- I. G. Dance and M. L. Scudder, J. Chem. Soc., Chem. Commun., 1995, 1039 RSC.
- I. Dance and M. Scudder, New J. Chem., 1998, 481 RSC.
- M. L. Scudder and I. G. Dance, J. Chem. Soc., Dalton Trans., 2000, 2909 RSC.
- I. G. Dance and M. L. Scudder, CrystEngComm, 2001, 12; http://www.rsc.org/ej/ce/2001/b100400j/index.htm Search PubMed.
- W. C. McCrone, in Polymorphism in Physics and Chemistry of the Organic Solid State, ed. D. Fox, M. M. Labes and A. Weissemberg, Interscience, New York, 1965, p. 726 Search PubMed.
- G. R. Desiraju, Crystal Engineering: The Design of Organic Solids, Elsevier, Amsterdam, 1989 Search PubMed.
- J. Bernstein, J. Phys. D: Appl. Phys., 1993, 26, B66 CrossRef CAS.
- T. L. Threlfall, Analyst, 1995, 120, 2435 RSC.
- J. D. Dunitz, Acta Crystallogr., Sect. B, 1995, 51, 619 CrossRef.
- J. D. Dunitz, in Perspectives in Supramolecular Chemistry: The Crystal as a Supramolecular Entity, ed. G. R. Desiraju, Wiley, Chichester, 1996, pp. 1–30 Search PubMed.
- D. Braga and F. Grepioni, Chem. Soc. Rev., 2000, 29, 229 RSC.
- C. Horn and I. G. Dance, 2001, unpublished results.
- L. K. Hansen, J. Sieler, P. Strauch, W. Dietzsch and E. Hoyer, Acta Chem. Scand. Ser. A, 1985, 39, 571 CrossRef.
- P. Strauch and L. Golic, Z. Anorg. Allg. Chem., 1996, 622, 1236 CrossRef CAS.
- L. Golic, N. Bulc and W. Dietzsch, Polyhedron, 1983, 2, 1201 CrossRef CAS.
- P. Strauch, W. Dietzsch, L. Golic, J. Sieler, A. Franke, I. Munzberg, K. Trubenbach, R. Kirmse, J. Reinhold and E. Hoyer, Inorg. Chem., 1995, 34, 1995 CrossRef.
- I. Dance and M. Scudder, Chem. Eur. J., 1996, 2, 481 CrossRef CAS PubMed.
- I. G. Dance and M. L. Scudder, J. Chem. Soc., Dalton Trans., 2000, 1587 RSC.
- G. R. Desiraju and T. Steiner, The Weak Hydrogen Bond in Structural Chemistry and Biology, Oxford University Press, Oxford, 1999 Search PubMed.
- The crystal structure CEFMIJ as originally reported had one phenyl C atom slightly misplaced: for the analysis here its position has been corrected and hydrogen atoms (which were reported as included, but which were not available from the CSD) added..
- I. G. Dance, in Perspectives in Supramolecular Chemistry: The Crystal as a Supramolecular Entity, ed. G. R. Desiraju, Wiley, Chichester, 1996, pp. 137–233 Search PubMed.
- V. M. Russell, M. L. Scudder and I. G. Dance, J. Chem. Soc., Dalton Trans., 2001, 789 RSC.
| This journal is © The Royal Society of Chemistry 2001 |