DOI:
10.1039/B100665G
(Paper)
CrystEngComm, 2001,
3, 60-63
A new coordination mode in oxalate-bridged polymers: molecular and crystal structure of [Cu3(C2O4)2(C2H8N2)4(ClO4)2]n·2H2O
Received
17th January 2001
, Accepted 15th February 2001
Abstract
The molecular and crystal structures of the title compound are reported. The molecular structure shows an oxalate-linked copper(II) two-dimensional coordination polymer where the knots are occupied by the [Cu(oxalate)2]n units and the edges by the [Cu(ethylenediamine)2]2n units which grow in the (011) plane. The compound crystallizes in the monoclinic system, space group P21/n, with a⊕=⊕15.579(3), b⊕=⊕8.073(2) and c⊕=⊕12.980(3) Å, β⊕=⊕118.67(2)°, Z⊕=⊕4, V⊕=⊕1432.3(6) Å3. The crystal structure is formed by a three-dimensional network via an extensive hydrogen-bonding network involving water molecules, nitrogen atoms from the ethylenediamine ligand and oxygen atoms from the perchlorate anions.
Introduction
Recently,1 carboxylate-bridged ligands have been reported in a number of applications, for example, as potential zeolites,2 and for their magnetic,3 conducting4 and non-linear optical properties.5 Research performed on several metals (alkali and alkaline earth, transition, rare earth and actinoid elements) with these ligands has provided an insight into these new properties.6 On the other hand, the fascinating structural variety formed through cooperative hydrogen-bonding interactions results in new crystalline architectures and it is these hydrogen-bonding interactions that play an important role in biological processes.7
Our investigations have thus been focused on extended solid frameworks with malonic acid. This research8 encouraged us to look also at biologically important dicarboxylic acids,7 such as oxalic acid. There is a wide variety of topologies for the coordination geometries of oxalic acid with different metal ions (Scheme 1). The aim of this research is to present a new coordination mode for oxalato-bridged copper(II) complexes (Scheme 2), leading to the 2D-polymeric compound [Cu3(C2O4)2(C2H8N2)4(ClO4)2]n·2H2O which was characterized by single crystal X-ray structure analysis.
 |
| | Scheme 1
| |
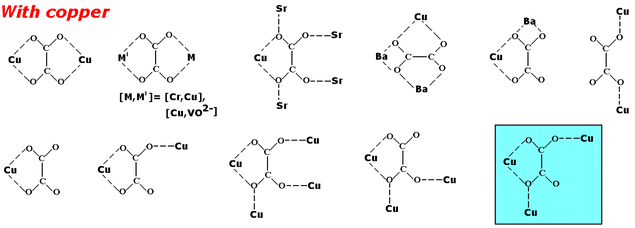 |
| | Scheme 2
| |
Experimental
General procedures and instrumentation
Copper perchlorate hexahydrate, ethylenediamine and sodium oxalate were used as received without further purification. Elemental analyses (C, H, N) were performed with an EA 1108 CHNS-O automatic analyser.
Synthesis
An aqueous solution of Na2C2O4 (5 mg, 4 mmol) in water (150 mL) was added to a solution of copper(II) perchlorate hexahydrate (30 mg, 8 mmol) in deionized water (50 mL). The mixture was stirred and a solution of ethylenediamine (4.8 mg, 8 mmol) was added dropwise with stirring. The solution was allowed to cool to room temperature and the white, insoluble precipitate that appeared after one week was separated by filtration. From the blue filtrate, blue crystals suitable for X-ray diffraction (Table 1) were collected after several days. Anal.: calc. for C12H36N8O18Cl2Cu3: C, 17.10; H, 4.27; N, 13.30. Found: C, 16.99; H, 3.39; N, 13.03%.
| Chemical formula |
C12H36N8O18Cl2Cu3 |
|
Click b100665g.txt for full crystallographic data (CCDC 157829).
|
| Formula weight |
842.014 |
| Crystal system |
Monoclinic |
| Space group |
P21/n |
|
a/Å |
15.579(3) |
|
b/Å |
8.073(2) |
|
c/Å |
12.980(3) |
|
β/° |
118.67(2) |
|
V/Å3 |
1432.3(6) |
|
Z
|
4 |
|
T/K |
293(2) |
|
ρ
calcd/g cm−3 |
1.943 |
|
μ/cm−1 |
24.8 |
| Measured independent/[I⊕>⊕σ(I)] reflections |
1638/1554 |
|
R
|
0.0537 |
|
R
w
|
0.1389 |
Crystallographic data collection and structure determination
A prismatic blue crystal of dimensions 0.40⊕×⊕0.30⊕×⊕0.27 mm3 was used for data collection on an Enraf-Nonius CAD-4 four-circle diffractometer. Data were collected at 293(2) K using graphite-monochromatized MoKα radiation (λ⊕=⊕0.71069 Å) and ω-scan techniques. Details of the crystal structure are given in Table 1. Lorentz, polarization and absorption corrections were made to the data.9 The maximum and minimum transmission factors were 1.341 and 0.775, respectively. The structure was solved using SIR9210 and refined using SHELXL93.11 In the final least squares cycles all non-hydrogen atoms were allowed to vibrate anisotropically. Hydrogen atoms were included at calculated positions on carbon centres. Hydrogen atoms attached to oxygen atoms of water molecules were not found. The final geometrical calculations and the graphical manipulations were carried out with PARST9512 and PLATON13 programs, respectively.
Results and discussion
Description of the structure
A perspective view of the asymmetric unit showing the atom-numbering scheme is given in Fig. 1. The three copper ions are located on crystallographic symmetry centres.
 |
| | Fig. 1
A perspective view of the asymmetric unit
| |
The three copper ions show a 4⊕+⊕2 coordination. In the [Cu(C2O4)2] units the oxalate groups occupy the basal positions with the copper–oxygen bonds nearly identical [1.932(6) and 1.930(7) Å for Cu(3)–O(1) and Cu(3)–O(2), respectively], while the apical positions are occupied by oxygen from the perchlorate anions [2.856(14) Å for Cu(3)–O(11)]. In the [Cu(en)2] units the basal plane is occupied by four nitrogen atoms from the ethylenediamine ligand in the basal plane, while the apical positions are occupied by oxygen from the oxalate groups. The bond lengths between copper and equatorial nitrogen are practically identical [2.004(7), 2.008(7), 2.011(6) and 2.019(11) Å for Cu(1)–N(1), Cu(2)–N(2), Cu(2)–N(3) and Cu(2)–N(4), respectively], and shorter than those between copper and the axial oxygen atoms [2.698(8) and 2.589(6) Å for Cu(1)–O(4) and Cu(2)–O(1), respectively].
In the three coordination spheres a strong Jahn–Teller effect is observed, the difference between the average basal bond lengths and the apical bond length are 0.612, 0.574 and 0.925 Å for Cu(1), Cu(2) and Cu(3), respectively. For Cu(3), the dihedral angle between the basal plane and the plane determined by the oxalate group is 6.2(2)°, and the deviation of the copper ions in respect to this former plane is 0.1522(2) Å (Table 2).
Table 2
Selected bond lengths (Å) and angles (°)
| Cu(1)–O(4) |
2.698(8) |
Cu(1)–N(1) |
2.004(7) |
N(3)–Cu(2)–N(4) |
84.6(5) |
| Cu(2)–O(1) |
2.589(6) |
Cu(1)–N(2) |
2.008(7) |
O(1)–Cu(2)–N(3) |
87.0(4) |
| Cu(3)–O(1) |
1.932(6) |
Cu(2)–N(3) |
2.011(6) |
O(1)–Cu(2)–N(4) |
87.6(3) |
| Cu(3)–O(2) |
1.930(7) |
Cu(2)–N(4) |
2.019(11) |
O(1)–Cu(3)–O(2) |
84.7(2) |
| Cu(3)–O(11) |
2.856(14) |
N(1)–Cu(1)–N(2) |
85.1(3) |
Cu(2)–O(1)–Cu(3) |
125.8(3) |
The oxalate ion adopts a new mode of coordination (see Fig. 2 and Scheme 2), simultaneously bidentate [at Cu(3)] and bis-monodentate [at Cu(1) and Cu(2)] exhibing a twist conformation. The average C–O and C–C bond lengths are 1.253(12) and 1.579(13) Å, respectively. The Cu⋯Cu separations are Cu(3)⋯Cu(2) 4.0365(10) Å [through the O(1) of the carboxylate group], Cu(1)⋯Cu(3) 6.4900(15) Å
(through the other carboxylate group) and Cu(1)⋯Cu(2) 7.6429(14) Å
(through the oxalate skeleton).
 |
| | Fig. 2
View of the oxalate coordination. The thermal ellipsoids are drawn at 50% probability level.
| |
The ethylenediamine ligands are not planar [maximum square deviation of atoms 0.403(12) Å]. The average C–C and C–N bond lengths are 1.491(17) and 1.480(12) Å, respectively.
The molecuar structure is a 2D polymer. The structure can be viewed as a net in which the knots are occupied by the [Cu(C2O4)2] units and the edges by the [Cu(en)2] units (Fig. 3). The crystal structure is formed by a 3D network through extensive hydrogen bonding involving the water molecules, ethylenediamine nitrogen atoms and perchlorate anion oxygen atoms (see Fig. 4 and Table 3).
 |
| | Fig. 3
A view of a fragment of the layer. The perchlorate and water molecules have been ommited for clarity.
| |
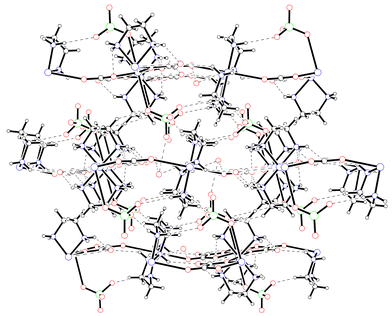 |
| | Fig. 4
Projection of the 3D hydrogen-bonded structure down the b axis. Dashed lines represent hydrogen-bonded interactions. Click image or 4.htm to access a 3D representation.
| |
Table 3
Selected donor–acceptor distances D⋯A (Å), angles⊕<⊕D⋯A (°) and O(water)⋯O distances (Å)
| D⋯A |
<D⋯A |
D⋯A |
<D⋯A |
| Symmetry codes: a⊕=⊕1⊕−⊕x, 1⊕−⊕y, 1⊕−⊕z; b⊕=⊕0.5⊕−⊕x, 0.5⊕+⊕y, 0.5⊕−⊕z; c⊕=⊕x, y⊕−⊕1, z; d⊕=⊕0.5⊕+⊕x, 1.5⊕−⊕y, 0.5⊕+⊕z. |
| N(1)–H(11)⋯O(3) |
2.254(14) |
141.4(9) |
N(2)–H(12)⋯O(13b) |
2.221(16) |
169.6(9) |
| N(1)–H(21)⋯O(1wa) |
2.276(11) |
155.1(8) |
N(3)–H(13)⋯O(3) |
2.260(12) |
146.4(9) |
| N(2)–H(22)⋯O(10) |
2319(12) |
152.5(9) |
N(3)–N(23)⋯O(2c) |
2.063(10) |
156.4(9) |
| O(water)⋯O |
| O(1w)⋯O(4) |
2.879(10) |
|
O(1w)⋯O(12d) |
2.948(11) |
|
Acknowledgements
Financial support from the Consejería de Educación and Cultura y Deportes from the Gobierno Autónomo de Canarias (Projects PI1999/061 and PI2000/135) is gratefully acknowledged.
References
-
(a) A. J. Blake, N. R. Champness, P. Hubberstey, W. S. Li, M. A. Withersby and M. Schöder, Coord. Chem. Rev., 1999, 183, 117 CrossRef CAS;
(b) D. Braga, F. Grepioni and G. R. Desiraju, Chem. Rev., 1998, 98, 1375 CrossRef CAS PubMed;
(c) Y. D. Posner, S. Dahal and I. Goldberg, Chem. Commun., 2000, 585 RSC.
-
(a) M. Munakata, L. P. Wu and T. K. Sowa, Adv. Inorg. Chem., 1999, 46, 173 CrossRef;
(b) C. Janiak, Angew. Chem., Int. Ed. Engl., 1997, 36, 1431 CrossRef CAS.
-
(a) O. Kahn and C. J. Martinez, Science, 1998, 279, 44 CrossRef CAS;
(b) O. Sato, T. Iyoda, A. Fujishama and K. Hashimoto, Science, 1996, 271, 49 CrossRef CAS.
- M. Munakata, C. L. Ning, T. K. Sowa, M. Maekawa, Y. Suenaga and T. Horino, Inorg. Chem., 1998, 37, 5651 CrossRef CAS PubMed.
-
(a) W. Lin, Z. Wang and L. Ma, J. Am. Chem. Soc., 1999, 121, 11249 CrossRef CAS;
(b) Y. K. Shan, R. H. Huang and S. D. Huang, Angew. Chem., Int. Ed., 1999, 38, 1751 CrossRef CAS.
-
(a) J. G. Kang, S. K. Yoon, Y. Sohn, J. G. Kim, Y. D. Kim and II. H. Suh, J. Chem. Soc., Dalton Trans., 1999, 1467 RSC;
(b) I. G. de Muró, F. A. Mautner, M. Insausti, L. Lezama, M. I. Arriortua and T. Rojo, Inorg. Chem., 1998, 37, 3243 CrossRef.
-
(a) J. Novasad, A. C. Messimeri, C. D. Papadimitriou, P. G. Veltsistas and J. D. Woollins, Transition Met. Chem., 2000, 25(6), 664 CrossRef and references therein;
(b) A. S. Batsanov, P. Hubberstey, C. E. Russell and P. H. Walton, J. Chem. Soc., Dalton Trans., 1997, 2667 RSC and references therein;
(c) I. Castro, J. Faus, M. Julve, M. C. Muñoz, W. Diaz and X. Solans, Inorg. Chim. Acta, 1991, 179, 59 CrossRef CAS.
-
(a) Ma. Hernández-Molina, P. A. Lorenzo-Luis, T. López, C. Ruiz-Pérez, F. Lloret and M. Julve, CrystEngComm, 2000, 31 Search PubMed;
(b) C. Ruiz-Pérez, J. Sanchiz, Ma .Hernández-Molina, F. Lloret and M. Julve, Inorg. Chem., 2000, 39, 1363 CrossRef;
(c) C. Ruiz-Pérez, Ma. Hernández-Molina, P. A. Lorenzo-Luis, F. Lloret, J. Cano and M. Julve, Inorg. Chem., 2000, 39, 3845 CrossRef;
(d) C. Ruiz-Pérez, J. Sanchiz, Ma. Hernández-Molina, F. Lloret and M. Julve, Inorg. Chim. Acta, 2000, 298, 202 CrossRef;
(e) C. Ruiz-Pérez, Ma .Hernández-Molina, J. Sanchiz, T. López, F. Lloret and M. Julve, Inorg. Chim. Acta, 2000, 298, 245 CrossRef;
(f)
Ma .Hernández-Molina, P. A. Lorenzo-Luis, C. Ruiz-Pérez, F. Lloret and M. Julve, Inorg. Chim. Acta, 2001, in press. Search PubMed;
(g)
Y. Rdguez-Martín, C. Ruiz-Pérez, J. Sanchiz, F. Lloret and M. Julve, Inorg. Chim. Acta, 2001, accepted for publication. Search PubMed;
(h)
J. Sanchiz Y. Rdguez-Martín, A. Mederos, C. Ruiz-Pérez, F. Lloret and M. Julve, Inorg. Chem., 2001, accepted for publication. Search PubMed;
(i)
Y. Rdguez-Martín, J. Sanchiz, C. Ruiz-Pérez, F. Lloret and M. Julve, J. Chem. Soc., Dalton Trans., 2001, accepted for publication. Search PubMed.
-
J. González-Platas and C. Ruiz-Pérez, NEWCORR: Program for Empirical Correction, University of La Laguna, Spain, 1997..
- A. Altomare, M. C. Burla, M. Camilla, G. Cascarano, C. Giacovazzo, A. Guagliardi and G. Polidori, J. Appl. Crystallogr., 1994, 27, 435 Search PubMed.
-
G. M. Sheldrick, SHELXL93, Program for the Refinement of Crystal Structures, University of Göttingen, Germany, 1993..
-
M. Nardelli, PARST95 (an update to PARST): a system of Fortran routines for calculating molecular structure parameters from the results of crystal structure analyses, J. Appl. Crystallogr., 1995, 28, 659. Search PubMed.
- A. L. Spek, Acta Crystallogr., Sect. A, 1990, 46, 34 Search PubMed.
|
| This journal is © The Royal Society of Chemistry 2001 |
Click here to see how this site uses Cookies. View our privacy policy here. 





