Selective anion binding and solid-state host–guest chemistry of an extended cavity calix[6]pyrrole
Boaz Turnera, Alexander Shterenberga, Moshe Kapona, Kinga Suwinskab and Yoav Eichen*a
aDepartment of Chemistry, Technion–Israel Institute of Technology, Technion City, 32000, Haifa, Israel.. E-mail: chryoav@tx.technion.ac.il
bInstitute of Physical Chemistry, Polish Academy of Sciences, Kasprzaka 44/52, PL-01 224, Warszawa, Poland
cSolid-State Institute, Technion–Israel Institute of Technology, Technion City, 3200, Haifa, Israel
First published on 19th December 2000
Abstract
Easily prepared, cone-like, extended cavity calix[6]pyrrole is shown to form strong complexes with iodine and other halide ions as well as with trihaloalkanes and electron deficient aromatic systems.
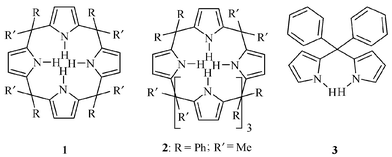
The host–guest chemistry of calixpyrroles has been the subject of intensive research aimed at gaining both understanding and control on guest recognition and binding.1 Recently, Sessler and coworkers2 reported that calix[4]pyrroles 1 are effective and selective receptors for anions and neutral guest species. Calixpyrroles that have been tailored to the size, shape and binding modes of target guests were shown to exhibit improved binding ability.3 Here we report the selective binding of anions, haloalkanes and aromatic guests by recently reported, cone-like, extended-cavity 1,1,3,3,5,5-meso-hexaphenyl-2,2,4,4,6,6- meso-hexamethylcalix[6]pyrrole.2,4 Calix[6]pyrroles are a new class of molecules characterized by a six-pyrrolemethane rim. The cross section of the hexapyrrolemethane rim is significantly larger than that of calix[4]pyrroles, ca. 110 cf. 24 Å2. Additionally, in the stable conformation of calix[6]pyrroles, such as 2, three of the meso phenyl substituents form a trigonal cavity with a volume of ca. 500 Å3. Such extended-cavity receptors may allow efficient and selective binding of electron defficient aromatic guests as well as large anions such as I−. These substrates can not be efficiently recognized nor complexed by the smaller calix[4]pyrrole systems.3b,5
Calix[6]pyrrole 2 was prepared using a modification of a previously reported method.4a Benzophenone (5 g, 27.4 mmol), pyrrole (5 mL, 72.3 mmol) and BF3·OEt (5 mL, 39.5 mmol) were dissolved in dry ethanol (250 mL) and stirred at room temperature for 5 days under an inert atmosphere. The precipitate was then filtered off and washed with cold ethanol, offering di(phenyl)di(pyrrol-2-yl)methane 3 as a colorless solid (40% yield). An additional crop of 3 could be isolated from the mother-liquor using column chromatography [silica, dichloromethane–hexane (1∶10), 50% total yield for 3]. Calix[6]pyrrole 2 was obtained by stirring a solution of 3 (300 mg, 1 mmol) in 60 mL of dry acetone–ethanol (1∶1) containing CF3CO2H for 5 days under inert atmosphere. The product was isolated by filtration (43% yield). An additional crop of 2 could be isolated from the mother-liquor using column chromatography [silica, dichloromethane–hexane (3∶7), 52% total yield for 2].
Similarly to earlier studies by Sessler and coworkers on the complexation of calix[4]pyrroles with different guest species, proton NMR spectroscopy was found to be a useful tool for the determination of binding constants between different guests and calix[6]pyrrole, 2.6 Quantitative assessments of anion binding constants were made by following the induced shifts in the 1H NMR spectra of the host as a function of the concentration of the guest in an acetonitrile–chloroform (1∶9) solution at room temperature (298 K). Table 1 lists the association constants between 2 and the different guests. For comparison, the complexation of octamethylcalix[4]pyrrole with the same guest species was studied under the same conditions (Table 1).
| Guest | Calix[4]pyrrole | Calix[6]pyrrole |
|---|---|---|
| F− | 23800 | 1080 |
| Cl− | 6800 | 650 |
| Br− | 270 | 50 |
| I− | <10 | 6600 |
| SCN− | <10 | <10 |
| p-MeC6H4SO3 − | <10 | 150 |
| BF4− | <10 | 2350 |
| MeCO2− | 400 | <10 |
| CF3CO2− | 70 | 1150 |
| EtOH | <10 | <10 |
| CF3CH2OH | <10 | 80 |
| CCl3CH2OH | <10 | 60 |
In accordance with previous studies performed by Sessler and coworkers octamethylcalix[4]pyrrole displays a clear preference towards fluoride ions over larger anions, the binding order being F− >> Cl− > Br− > I−. In contrast, probably due to its extended cavity, 2 exhibits a clear preference to iodide over smaller halides. Here the binding order switches to I− > F− >> Cl− > Br−. We interpret this binding order and the high affinity towards iodide in terms of the geometrical fit between the extended cavity of 2 and the iodide ion, allowing full binding of the anion by up to six pyrrole rings. Being the smallest halide, the fluoride ion may fit and bind efficiently to only part of the pyrrolemethane ring. Similar effects have previously been reported for the binding of cations to the cavities of crown ethers.7
Calix[6]pyrrole 2 is found to bind also to trihalogenated species such as trichloroethanol, trifluoroethanol, tetrafluoroborate and trifluoroacetate and forms significantly stronger complexes with such guests than with their nonhalogenated ananolgs (Table 1). The reason for this is revealed from the crystal structure† of such a complex between 2,2,2-trichloroethanol and 2 (Fig. 1). Unlike simple calixpyrroles that bind their guests predominantly through X−⋯H–N bonds,2,3b2 anchors the 2,2,2-trichloroethanol guest through one (disordered) H–O⋯H–N hydrogen bond with the hydroxy group, d(O84b⋯H–N18) = 2.411 Å; α(O–H–N) = 169.11°; d(O84a⋯H–N70) = 2.412 Å; α(O–H–N) = 175.95°; d(O84a⋯H–N44) = 2.725 Å; α(O–H–N) = 156.97°, and three πphenyl⋯Cl–C bonds between the Cl atoms of the guest and the π electron clouds of the three axial meso-phenyl groups forming the pseudo-threefolded cavity of 2: d(Cl79–π(C34–C39)) = 3.38 Å; d(Cl80–π(C60–C65)) = 3.00 Å; d(Cl81–π(C8–C13)) = 3.45 Å.
![The molecular structure of the complex between 2,2,2-trichloroethanol
and calix[6]pyrrole 2. Solvent and other molecules not situated in
the cavity of the host have been omitted for clarity.](/image/article/2001/CC/b007788g/b007788g-f1.gif) | ||
| Fig. 1 The molecular structure of the complex between 2,2,2-trichloroethanol and calix[6]pyrrole 2. Solvent and other molecules not situated in the cavity of the host have been omitted for clarity. | ||
The stable conformation of 2 brings two electron-rich pyrrole rings, situated in a 1,4 position to one another, into a parallel and cofacial orientation. These two rings are spaced ca. 7.1 Å apart. Being an electron rich ring system, the hexapyrrolemethane ring is suitable for hosting electron poor conjugated species in between a pair of cofacial pyrrole rings. The additional four pyrrole rings are capable of forming multiple hydrogen bonds with appropriate guests, making the system an interesting host for different nitro- and carboxy-aromatic compounds. Fig. 2 shows the crystallographic structure† of a complex between p-nitrotoluene/nitrobenzene and 2. Interestingly, though crystallized from a solution containing nitrobenzene and p-nitrotoluene in a 10∶1 ratio, the crystal structure clearly indicates the 1∶1 inclusion of nitrobenzene and p-nitrotoluene within the cavity of 2. As can be seen in Fig. 2, the nitroaromatic guest is fixed to the cavity of the host through short range π–π interactions between the nitro group of the guest and the two sandwiching pyrrole rings of the host, d(nitro(plane)⋯pyrrole(plane)) = 3.55 Å. Three of the other four pyrrole rings are involved in hydrogen bonding with the nitro group of the encapsulated guest, d(NH1⋯O80) = 2.23 Å, d(N1⋯O80) = 3.06 Å, α(O–H–N) = 172.94°, d(NH31⋯O80) = 2.38 Å, d(N31⋯O80) = 3.18 Å, α(O–H–N) = 143.67°, d(NH19⋯O81) = 2.45 Å, d(N19⋯O81) = 3.23 Å, α(O–H–N) = 165.11°.
![The molecular structure of the complex between calix[6]pyrrole and
nitrotoluene/nitrobenzene. Molecules that are not situated in the cavity of
the host have been omitted for clarity.](/image/article/2001/CC/b007788g/b007788g-f2.gif) | ||
| Fig. 2 The molecular structure of the complex between calix[6]pyrrole and nitrotoluene/nitrobenzene. Molecules that are not situated in the cavity of the host have been omitted for clarity. | ||
In conclusion, calix[6]pyrrole 2 shows a wealth of binding modes to different substrates, ranging from simple anions to aromatic derivatives. The axial meso phenyl groups form a genuine preorganized cavity and actively participate in binding trihalogenated compounds. The application of calix[6]pyrroles to the separation and identification of such compounds is under investigation.
Acknowledgements
This work was supported by the Israel Science Foundation, Administrated by the Israel Academy of Sciences and Humanities and by the Fund for the Promotion of Sponsored Research at the Technion.Notes and referenecs
- J. M. Lehn, Supramolecular Chemistry, Concepts and Prespectives, VCH, Weinheim, 1995. Search PubMed.
- P. A. Gale, J. L. Sessler, V. Kral and V. Lynch, J. Am. Chem. Soc., 1996, 118, 5140 CrossRef CAS.
- (a) P. Anzenbacher, Jr., K. Jursikova, V. M. Lynch, P. A. Gale and J. L. Sessler, J. Am. Chem. Soc., 1999, 121, 11020 CrossRef; (b) G. Cafeo, F. H. Kohnke, G. L. La Torre, A. J. P. White and D. J. Williams, Chem. Commun., 2000, 1207 RSC; (c) S. Camiolo and P. A. Gale, Chem. Commun., 2000, 1129 RSC.
- (a) B. Turner, M. Botoshansky and Y. Eichen, Angew. Chem., Int. Ed., 1998, 37, 2475 CrossRef CAS; B. Turner, M. Botoshansky and Y. Eichen, Angew. Chem., 1998, 110, 2633 CrossRef; (b) for an alternative approach to calix[6]pyrroles see: G. Cafeo, F. H. Kohnke, G. L. La Torre, A. J. P. White and D. J. Williams, Angew. Chem., Int. Ed., 2000, 39, 1496 CrossRef CAS.
- P. A. Gale, L. J. Twyman, C. I. Handlin and J. L. Sessler, Chem. Commun., 1999, 1851 RSC.
- W. E. Allen, P. A. Gale, C. T. Brown, V. M. Lynch and J. L. Sessler, J. Am. Chem. Soc., 1996, 118, 12471 CrossRef CAS; H. Miyaji, P. Anzenbacher, J. L. Sessler, E. R. Bleasdale and P. A. Gale, Chem. Commun., 1999, 1723 RSC; P. A. Gale, J. L. Sessler and V. Kral, Chem. Commun., 1998, 1 RSC; P. A. Gale, J. L. Sessler, W. E. Allen, N. A. Tvermoes and V. Lynch, Chem. Commun., 1997, 665 RSC.
- A. Alvanipour, J. L. Atwood, S. G. Bott, P. C. Junk, U. H. Kynast and H. Prinz, J. Chem. Soc., Dalton Trans., 1998, 1223 RSC; J. L. Atwood, S. G. Bott, S. Harvey and P. C. Junk, Organometallics, 1994, 13, 4151 CrossRef CAS.
- Nonius, KappaCCD Server software. Nonius BV, Delft, The Netherlands..
- Z. Otwinowski and W. Minor, Methods Enzymol., 1997, 276, 307 Search PubMed.
- G. M. Sheldrick, Acta Crystaltogr. Sect. A, 1990, 46, 467 CrossRef.
- G. M. Sheldrick, SHELXL97, University of Göttingen, Germany..
Footnote |
| † Crystal data: for
2·2.5CCl3CH2OH·1.5CHCl3
·3CH3CH2OH·5.5H2O:
grown in the dark from
2,2,2-trichloroethanol–chloroform–ethanol. A single crystal was
mounted on the Nonius Kappa CCD diffractometer,8 and cooled to 170 K under a nitrogen stream. Data
were collected with graphite-monochromated Mo-Kα radiation
(λ = 0.71070 Å) by applying φ and
ω rotations. Data reduction was performed using DENZO-SMN
software.9 The structure was solved using
direct methods (SHELXS-9710) and refined by
SHELXL-97.11 All non-H atoms of the
macrocycle and the trichloroethanol inside the cavity, excluding the
disordered hydroxy oxygen, were refined anisotropically. Hydrogen atoms of
these moieties were placed at calculated positions and refined as riding on
their carbon and nitrogen atoms. Difference Fourier maps based on the
macrocycle and the guest inside, revealed another moderately disordered
trichloroethanol bound to the macrocycle outside the cavity, and another
four sites of severely disordered molecules such as trichloroethanol,
chloroform, ethanol and water. All the disordered positions of the solvent
molecules were refined isotropically. 38 hydrogen atoms belonging to some
of the disordered solvent molecules were not allocated.
Mr = 1804.83, monoclinic, space group
P21/n, a = 17.773(10), b =
20.2090(10), c = 26.2590(10) Å, β =
107.730(3)°, V = 8983.6(8) Å3, T =
170.0(1) K, Z = 4, μ = 0.076 mm−1,
14385 relections measured, 14385 unique which were used in all
calculations. The final R(F2) was
0.1168 [I > 2σ(I)]. For 2·2.5C6H5NO2·0.5C 7H9NO2: grown in the dark by slow evaporation of a chloroform solution. A single crystal was mounted on the Nonius Kappa CCD diffractometer, at 293 K. Data collection and reduction as above. The structure was also solved and refined as above. All non-H atoms of the macrocycle and the guests were refined anisotropically. Hydrogen atoms were placed at calculated positions and refined as riding on their carbon and nitrogen atoms except for the N–H hydrogen atoms of the pyrrole rings which were localized on a Fourier difference map and refined isotropically. Mr = 1392.66, monoclinic, space group P21/n, a = 19.455(1), b = 19.762(1), c = 22.027(1) Å. β = 115.405(2)°, V = 7649.8(5) Å3, T = 293 K, Z = 4, μ = 0.076 mm−1, 16018 relections measured, 15694 unique which were used in all calculations. The final R(F2) was 0.0740 [I > 2σ(I)]. CCDC 182/1864. See http://www.rsc.org/suppdata/cc/b0/b007788g/ for crystallographic files in .cif format. |
| This journal is © The Royal Society of Chemistry 2001 |