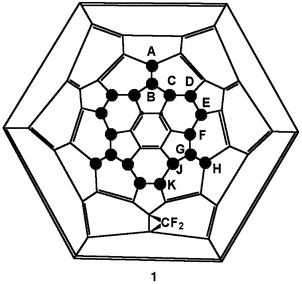
Isolation and spectroscopic characterisation of C60F18CF2, the first difluoromethano[60]fullerene
Anthony G. Aventa, Olga V. Boltalinab, Andrei Yu Lukoninb, Joan M. Streetc and Roger Taylora
aThe Chemistry Laboratory, CPES School, University of Sussex, Brighton, UK BN1 9QJ
bChemistry Department, Moscow State University, Moscow, 119899, Russia
cChemistry Department, The University, Southampton, UK SO17 1BJ
First published on UnassignedUnassigned23rd December 1999
Abstract
From the fluorination of [60]fullerene by K2PtF6 at 465 °C under 0.1 bar, we have isolated and characterised C60F18CF2 which has Cs symmetry and is isostructural with C60F18O; it co-elutes (HPLC) with a minor unsymmetrical isomer.
Numerous methano[60]fullerenes C60CR1R2 have been prepared by methods which include the addition of carbenes or their diazo precursors, of α-halocarbanions, and the use of ylides.1 1′,1′-Dihalofullerenes C60CX2, have been formed either by pyrolysis of sodium chloroacetate in the presence of the fullerene (X2 = Cl2),2 by generating a dihalocarbene from the haloform in the presence of a base (X2 = Br2, BrCl, I2),3 or by reacting the fullerene with PhHgCBr3, X2 = CBr2.4 However, no compounds with X2 = F2 are known, but mass spectrometry of fluorofullerenes has indicated that the CF2 moiety may either be present in some mixtures of compounds, or is produced by fragmentation.5 In a report describing HPLC purification of C60F18O, a component of 1112 amu (corresponding to C60F18CF2) was detected in one fraction of the eluent.6 We have now succeeded in isolating this component and characterising it fully.
[60]Fullerene (240 mg) was ground in a dry box with K2PtF6 (575 mg) and heated to 465 °C at ca. 0.01 bar in a glass tube contained within a furnace, as described previously.6 The crude fluorofullerene mixture (300 mg, 85%) was partly prepurified by vacuum sublimation and a sample (ca. 280 mg) was dissolved in dry toluene (25 ml) and filtered under conditions which avoided moisture condensation. Purification by HPLC (10 × 250 mm Cosmosil Buckyprep column) with toluene elution at a flow rate of 4.7 ml min−1 (![[triple bond, length as m-dash]](https://www.rsc.org/images/entities/char_e002.gif) 1 ml min−1 for a 4.6 mm diameter column), yielded recovered [60]fullerene (ca. 75 mg), C60F18
1 ml min−1 for a 4.6 mm diameter column), yielded recovered [60]fullerene (ca. 75 mg), C60F18![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) 5 (ca. 100 mg) together with twenty other components in 1–5 mg yields, including C60F18O, described previously.7
5 (ca. 100 mg) together with twenty other components in 1–5 mg yields, including C60F18O, described previously.7
The mass spectrum of the fraction (ca. 3 mg) eluting at 31 min (Fig. 1) shows the parent ion at 1112 amu (C60F18CF2), with very prominent fragmentation by initial CF3 loss. [This compound therefore elutes more rapidly than C60F18 and C60F18O (36.5 and 45 min, respectively).] We have found such fragmentation (but on a lesser scale) to be a feature of EI mass spectrometry of fluorofullerenes (the mechanism being unclear), but here it is evidently enhanced by the presence of the CF2 group. Moreover, the intensity of the 720 amu peak is much greater than is customary for fluorofullerenes and further indicates that the CF2 group accelerates the fragmentation process. The presence of 18 fluorines shows that the compound is a derivative of the known C60F18.
 | ||
| Fig. 1 EI mass spectrum (70 eV) for C60F18CF2. | ||
The origin of the CF2 group is indicated by the detection of numerous by-products containing both H and CF3 species in the above fluorination. CF3 radicals are produced by fragmentation of some of the cages as noted above, and these then attack other cages followed by the usual adventitious acquisition of hydrogen. The mass spectra of some (but not all) of the C60(H,CF3)n derivatives show loss of 20 amu (HF![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ), which arises evidently when the H and CF3 groups are adjacent, thereby producing the CF2 group.
), which arises evidently when the H and CF3 groups are adjacent, thereby producing the CF2 group.
The IR spectrum (bands at 1200, 1195, 1160, 1133, 1106, 1100 and 830 cm−1, Fig. 2) shows the same fine structure characteristics of C60F18 and C60F18O (cf. refs. 5,6). However, there are additional bands at 1282 and 1254 cm−1 characteristic of aliphatic C–F bonds.
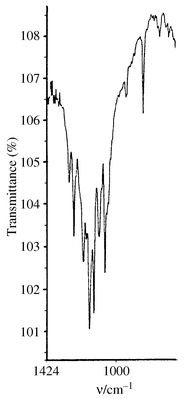 | ||
| Fig. 2 IR spectrum (KBr) for C60F18CF2. | ||
The 19F NMR spectrum (Fig. 3, 376.4 MHz) consists of eleven peaks (some coincident) at δF
−130.7 (2 F, d, 17.6 Hz), −131.53 (1 F, d, 15.5 Hz), −136.11 and −136.25 (overlapping multiplets, 6 F![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ), −137.82 (2 F, d, 17.8 Hz; additional minor peaks lie underneath here causing the integral to be approximately 25% too large—see below), −142.91 (2 F, m), −142.98 (4 F, m), −144.89 (2 F, d, 27 Hz), −157.50 (2 F, m) and −157.68 (1 F, m). Some minor peaks are also present and analysis (below) shows the product to consist of a mixture of a CS isomer and an unsymmetrical one in ca. 65∶35 ratio; the minor peaks (in approximately the same ratio) were reproduced in a second preparation.
), −137.82 (2 F, d, 17.8 Hz; additional minor peaks lie underneath here causing the integral to be approximately 25% too large—see below), −142.91 (2 F, m), −142.98 (4 F, m), −144.89 (2 F, d, 27 Hz), −157.50 (2 F, m) and −157.68 (1 F, m). Some minor peaks are also present and analysis (below) shows the product to consist of a mixture of a CS isomer and an unsymmetrical one in ca. 65∶35 ratio; the minor peaks (in approximately the same ratio) were reproduced in a second preparation.
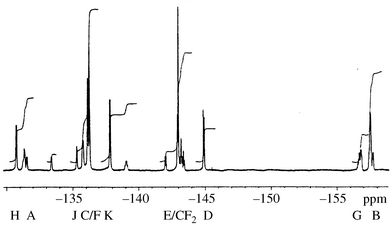 | ||
| Fig. 3 19F NMR spectrum (376.4 MHz) for C60F18CF2. | ||
The 2D 19F NMR spectrum (Fig. 4) reveals the structure of the CS isomer to be 1; there are only three positions that the CF2 group can occupy for a symmetrical structure and only one of them can give a singlet for the CF2 group.
![2D 19F NMR spectrum for C60F18CF2 [inset shows expansion of the −130 to −138 ppm region].](/image/article/2000/P2/a909157b/a909157b-f4.gif) | ||
| Fig. 4 2D 19F NMR spectrum for C60F18CF2 [inset shows expansion of the −130 to −138 ppm region]. | ||
(i) Previous 19F NMR data for fluorofullerenes have indicated that upfield peaks are due generally to fluorines attached to carbon having three adjacent sp3-hybridised carbon atoms, whilst downfield peaks are due to fluorines attached to carbons having only one adjacent sp3-hybridised carbon atom.7,8 This is due to the greater electronegativity of sp2 carbons compared to sp3 carbons.6 Thus for structure 1, there should be two upfield fluorines (G) coupled to two downfield fluorines (H), and likewise a single upfield fluorine (B) coupled to a single downfield fluorine (A). This is found (see inset to Fig. 4).
(ii) Fluorines G should be coupled to fluorines F and J, likewise fluorine B should be coupled to fluorines C, which locates these peaks in the spectrum (Fig. 4 inset). Note that fluorines C and F are coincident in the spectrum. Fluorines J are also coupled to fluorines K as required so these are also located. The 2D spectrum confirms that there are peaks underlying K (see above) and these are due to the minor isomer.
(iii) Fluorines D and E are coupled as required by the structure, but the integral for E is twice that for D due to the coincidence of the singlet due to the CF2 group. The appearance of the latter at −143 ppm is entirely consistent with literature values (−143.2, −142.7, −143.2 ppm) for CF2 forming part of a three-membered ring.9 The coupling between fluorines C and F could not be seen; likewise in the 2D 19F NMR spectrum for C60F18O the couplings between fluorines G and either fluorines F and J were not found.
(iv) Fluorines D and E can be distinguished because E is coupled with F.
(vii) Both C and F are nearer to the central benzenoid ring than are D and E, and the resonances appear more downfield, this same pattern being found previously for C60F18 and C60F18O,6,8 and may be a general feature for compounds of this type.
The minor isomer
The integrals for the fluorine content of the Cs isomer is 65% of the total observed. Those for the remainder, δF −131.2 to −131.4 (3 F, overlapping m), −133.38 (1 F, d), −135.28 (2 F, br s), −135.75 (3 F, br s), −139.1 (2 F, q), −142.05 (1 F, d), −143.19 (2 F, t), −143.36 (1 F, t), 156.5–156.9 (3 F, overlapping m), −157.4 (2 F, m), correspond to the presence of 20 fluorines (taking the smallest integral as 1 F![[hair space]](https://www.rsc.org/images/entities/char_200a.gif) ), indicating that an unsymmetrical isomer is also present. Moreover it shows the same characteristics as the major isomer in having coupling between the low- and high-field fluorines, but with a difference in that there are at least four low-field fluorines. There is only one reasonable position for the CF2 group to add, seen by reference to the labelled framework of 1, i.e., without the CF2 group shown. This is to add to the double bond (which has localised electrons) in the ring with the A–D labels (or the five other equivalents). This is energetically less favourable than the addition which produces 1, but is statistically favoured by a factor of 2∶1. Such an addition now causes the carbon bearing fluorine D to be surrounded by three sp3-hybridised carbons so that there are now four fluorines in such environments, requiring four upfield peaks.
), indicating that an unsymmetrical isomer is also present. Moreover it shows the same characteristics as the major isomer in having coupling between the low- and high-field fluorines, but with a difference in that there are at least four low-field fluorines. There is only one reasonable position for the CF2 group to add, seen by reference to the labelled framework of 1, i.e., without the CF2 group shown. This is to add to the double bond (which has localised electrons) in the ring with the A–D labels (or the five other equivalents). This is energetically less favourable than the addition which produces 1, but is statistically favoured by a factor of 2∶1. Such an addition now causes the carbon bearing fluorine D to be surrounded by three sp3-hybridised carbons so that there are now four fluorines in such environments, requiring four upfield peaks.OVB and RT thank the Royal Society for a Joint Project Award.
References
- For a recent summary, see R. Taylor , Lecture Notes on Fullerene Chemistry: A Handbook for Chemists, Imperial College Press, London, 1999, ch. 9. Search PubMed.
- M. Tsuda, T. Ishida, T. Nogami, S. Kurono and M. Ohashi, Tetrahedron Lett., 1993, 34, 6911 CrossRef CAS; Fullerene Sci. Technol., 1993, 1, 275; Search PubMed; 1994, 2, 155; ; J. Chem. Soc., Chem. Commun., 1993, 1296. Search PubMed.
- K. Komatsu, Y. Murata, A. Miyabo, K. Takeuchi and T. S. M. Wan, Fullerene Sci. Technol., 1993, 1, 231 CAS; T. Ishida, T. Furudate, T. Nogami, M. Kubota, T. Hirano and M. Ohashi, Fullerene Sci. Technol., 1995, 3, 399 CAS; A. M. Benito, A. D. Darwish, H. W. Kroto, M. F. Meidine, R. Taylor and D. R. M. Walton, Tetrahedron Lett., 1996, 1085 CrossRef CAS.
- J. Osterodt and F. Vögtle, Chem. Commun., 1996, 547 RSC.
- R. Taylor, G. J. Langley, J. H. Holloway, E. G. Hope, H. W. Kroto and D. R. M. Walton, J. Chem. Soc., Chem. Commun., 1993, 875 RSC; O. V. Boltalina, A. K. Abdul-Sada and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1995, 981 RSC.
- O. V. Boltalina, V. Yu. Markov, R. Taylor and M. P. Waugh, Chem. Commun., 1996, 2549 RSC.
- A. G. Avent, O. V. Boltalina, P. W. Fowler, A. Yu. Lukonin, V. K. Pavlovich, J. P. B. Sandall, J. M. Street and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1998, 1319 RSC.
- O. V. Boltalina, M. Bühl, A. Khong, M. Saunders, J. M. Street and R. Taylor, J. Chem. Soc., Perkin Trans. 2, 1999, 1475 RSC.
- J. W. Emsley and L. Philips , Progress in NMR Spectroscopy, eds. J. W. Emsley, J. Feeney and L. H. Sutcliffe, Pergamon Press, Oxford, Vol. 7, 1971, Table A13. Search PubMed.
| This journal is © The Royal Society of Chemistry 2000 |