Professor Patrick D. Bailey![[hair space]](https://www.rsc.org/images/entities/h2_char_200a.gif) †
†
First published on 31st August 2000
Career
Patrick Bailey was born in Derbyshire in 1959, and took his undergraduate degree in Chemistry at Oxford, where he remained to carry out a DPhil on peptide chemistry with Geoffrey Young. His time at Oxford was split between mountaineering/climbing/caving and chemistry (including acting as a college tutor at St. Catherine’s College), and at the end of his doctorate, he enrolled for a teacher training course in “Chemistry with Outdoor Pursuits.” Before he could start this, however, a lectureship at York lured him into academia, and he stayed at York until the end of 1992, when he took up the Chair of Organic Chemistry at Heriot-Watt University, Edinburgh. He received a Career Development Award from the Cancer Research Campaign in 1986, and was elected to FRSC in 1994 and FRSE in 1999. He received the Zeneca Research Award in Organic Chemistry in 1994, the Lord Kelvin Lectureship from the BAAS in 1999, an RSC HE Teaching Award in 1999, and is the RSC Nyholm Lecturer for 2000/2001. | ||
| Plate1 Professor Patrick D. Bailey | ||
Teaching
I have always believed fervently that teaching and research should run side-by-side at University, and I hope that one of my major contributions to HE so far has been to start to raise the status and importance of teaching at HE level. I have been involved in several such initiatives, of which I highlight four:1) University Chemistry Education is a RSC journal that was started in 1997 – one of several initiatives established by John Garratt. It publishes papers, communications, perspectives and reviews, allowing practising chemistry lecturers to disseminate innovative teaching methods in a recognized primary journal.
2) Variety in Chemistry Teaching is the HE chemistry teaching event of the year. There are around 100 attendees annually, with almost every chemistry department in the UK represented, and the programme includes participative workshops, international plenary lectures, short communications, and demonstrations/posters.
3) Project Improve has been a hugely successful HEFCE-funded programme run from Hull, which has delivered 10 booklets, 30 workshops, 15 short-term placements and a Web site. This has generated an amazing resource of expertise and material and, with the new Physical Sciences Subject Centre also based at Hull, this will continue to be a major HE teaching facility for chemistry.
4) Communicating Chemistry is the title of a module that we teach at Heriot-Watt, in which students develop a wide range of transferable skills in a chemistry context. Funded by an RSC Cutter Bequest, Sara Shinton and I produced a teaching manual of 10 exercises, and a student’s reference booklet that goes to all final year undergraduates in the UK.
Research
My organic research all involves work on biologically important molecules, focusing on 4 main sub-themes.a) Indole alkaloids
By a careful study of the mechanism of the Pictet–Spengler reaction, we have developed the only general (and asymmetric) route to cis-1,3-disubstituted tetrahydro-β-carbolines, starting from L-tryptophan (Scheme 1),1 and we have applied this to the synthesis of several natural products and analogues.2–10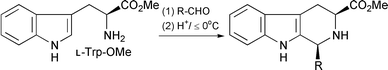 | ||
| Scheme 1 Pictet–Spengler reaction under conditions of kinetic control: yields are typically >90%, cis∶trans ratio ca. 4∶1, and ee > 95%. | ||
b) Substituted piperidines
The piperidine moiety is the most commonly occurring heterocyclic system amongst alkaloids, and we were the first to discover conditions under which imines of the type PhCH2N![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) CHCO2R could undergo aza-Diels–Alder reactions with acyclic dienes;11 this opened up a rapid, simple, and flexible route to a wide range of substituted piperidines, and we have now developed asymmetric routes to compounds of this type (Scheme 2).12–14
CHCO2R could undergo aza-Diels–Alder reactions with acyclic dienes;11 this opened up a rapid, simple, and flexible route to a wide range of substituted piperidines, and we have now developed asymmetric routes to compounds of this type (Scheme 2).12–14
 | ||
| Scheme 2 Asymmetric aza-Diels–Alder reactions (R = Et or 8-phenylmenthyl). Yields are typically ca. 50%, and the reaction shows total regio- and diastereo-control. For R = 8-phenylmenthyl, asymmetric induction is >95%, allowing the total asymmetric synthesis of several natural products. | ||
c) Polycyclic peptides
During 1990–1995, we prepared the first polycyclic peptides (PCPs) and showed that they would bind guests enantiospecifically (see Figs. 1 and 2).15 We extended the synthetic methodology to allow access to a wide range of PCPs,16–18 and we are currently preparing new PCPs as enzyme mimics. | ||
| Fig. 1 The structure of A is a bowl shape, in which guest molecules (e.g.B) can bind. | ||
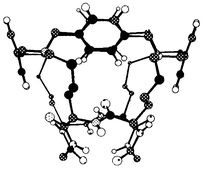 | ||
| Fig. 2 Structure of A from NMR data. | ||
d) Studies on peptide transport
In work on the PepT1 transporter protein, we are providing the synthetic input into a collaborative programme with colleagues at York and Oxford, probing the structural and mechanistic features of the PepT1 peptide transporter protein (Fig. 3) using a range of carefully designed di- and tri-peptides and peptide analogues.19–22 | ||
| Fig. 3 The template that substrates must adopt for transport by PepT1 (see ref. 22). | ||
My thanks go to my colleagues, research group, and (most!) undergraduates at Heriot-Watt and York who support my enjoyment of chemistry.
Teaching materials, resources and events
1) University Chemistry Education is published twice-yearly free to RSC members registered with the Education Division (which is also free). The journal will be on-line from 2001, and all issues can be accessed through the RSC Education Division Web page, or directly at: www.rsc.org/uchemed/uchemed.htm (Editor: John Garratt, email: cjg2@york.ac.uk).2) Variety in Chemistry Teaching in 2001 will be 17–18 September at Lancaster University – contact Stephen Breuer on s.breuer@lancaster.ac.uk, or enrol with ChemEducation Mailbase (via http://www.mailbase.ac.uk) to receive information on this and other HE matters.
3) Project Improve material can be obtained through the new Physical Sciences Centre: email: 1tsn-psc@hull.ac.uk; Web: http://www.physsci.1tsn.ac.uk; (Director: Tina Overton, email: t.1.overton@chem.hull.ac.uk)
4) Communicating Chemistry: a) P. D. Bailey and S. E. Shinton, Communicating Chemistry: A Teaching Manual for University Teachers, Royal Society of Chemistry, London, 1999, pp. 141. b) P. D. Bailey and S. E. Shinton, Getting the Message Across: Key Skills for Chemists, eds. D. Rafferty and A. Kumar, Royal Society of Chemistry, London, 1998, pp. 40. c) P. D. Bailey, Teaching Communication Skills in Chemistry, Univ. Chem. Educ., 1997, 1, 31.
References
- P. D. Bailey, S. P. Hollinshead, N. R. Mclay, K. M. Morgan, S. J. Palmer, S. N. Prince, C. D. Reynolds and S. D. Wood, J. Chem. Soc., Perkin Trans. 1, 1993, 431 RSC.
- P. D. Bailey and N. R. Mclay, J. Chem. Soc., Perkin Trans. 1, 1993, 441 RSC.
- P. D. Bailey, S. P. Hollinshead, N. R. Mclay, J. H. Everitt, C. D. Reynolds, S. D. Wood and F. Giordano, J. Chem. Soc., Perkin Trans. 1, 1993, 451 RSC.
- P. D. Bailey, M. H. Moore, K. M. Morgan, D. I. Smith and J. M. Vernon, Tetrahedron Lett., 1994, 35, 3587 CrossRef CAS.
- P. D. Bailey, I. D. Collier, S. P. Hollinshead, M. H. Moore, K. M. Morgan, D. I. Smith and J. M. Vernon, J. Chem. Soc., Chem. Commun., 1994, 1559 RSC.
- P. D. Bailey and K. M. Morgan, Chem. Commun., 1996, 1479 RSC.
- P. D. Bailey, I. D. Collier, S. P. Hollinshead, M. H. Moore, K. M. Morgan, D. I. Smith and J. M. Vernon, J. Chem. Soc., Perkin Trans. 1, 1997, 1209 RSC.
- (a) P. D. Bailey, P. J. Cochrane, F. Irvine, K. M. Morgan, D. P. J. Pearson and K. T. Veal, Tetrahedron Lett., 1999, 40, 4593 CrossRef CAS.
- P. D. Bailey, P. J. Cochrane, A. H. Forster, K. M. Morgan and D. P. J. Pearson, Tetrahedron Lett., 1999, 40, 4597 CrossRef CAS.
- P. D. Bailey, K. M. Morgan, G. M. Rodair and R. Ll. Thomas, Tetrahedron Lett., 1999, 40, 8255 CrossRef CAS.
- P. D. Bailey, R. D. Wilson and G. R. Brown, Tetrahedron Lett., 1989, 30, 6781 CrossRef CAS.
- P. D. Bailey, R. D. Wilson and G. R. Brown, J. Chem. Soc., Perkin Trans. 1, 1991, 1337 RSC.
- P. D. Bailey, G. R. Brown, F. Korber, A. Reid and R. D. Wilson, Tetrahedron: Asymmetry, 1991, 2, 1263 CrossRef CAS.
- P. D. Bailey, D. J. Londesbrough, T. C. Hancox, J. D. Heffernan and A. B. Holmes, J. Chem. Soc., Chem. Commun., 1994, 2543 RSC.
- P. D. Bailey, D. G. W. Clarke and G. A. Crofts, J. Chem. Soc., Chem. Commun., 1992, 658 RSC.
- P. D. Bailey and G. A. Crofts, Tetrahedron Lett., 1994, 35, 3207 CrossRef.
- P. D. Bailey, S. R. Carter, D. G. W. Clarke, G. A. Crofts, J. H. M. Tyszka, P. W. Smith and P. Ward, Tetrahedron Lett., 1994, 35, 3211 Search PubMed.
- P. D. Bailey, S. R. Carter, D. G. W. Clarke, G. A. Crofts, P. W. Smith and P. Ward, Tetrahedron Lett., 1994, 35, 3215 Search PubMed.
- C. S. Temple, P. D. Bailey, J. R. Bronk and C. A. R. Boyd, J. Physiol., 1996, 494, 795 CrossRef CAS.
- N. Lister, P. D. Bailey, I. D. Collier, C. A. R. Boyd and J. R. Bronk, Biochim. Biophys. Acta, 1997, 1324, 245 CrossRef CAS.
- C. S. Temple, A. K. Stewart, D. Meredith, N. A. Lister, K. M. Morgan, I. D. Collier, R. D. Vaughan-Jones, C. A. R. Boyd, P. D. Bailey and J. R. Bronk, J. Biol. Chem., 1998, 273, 18 CrossRef.
- P. D. Bailey, C. A. R. Boyd, J. R. Bronk, I. D. Collier, D. Meredith, K. M. Morgan and C. S. Temple, Angew. Chem., Int. Ed., 2000, 39, 506 CrossRef CAS.
Footnote |
| † Department of Chemistry, Heriot-Watt University, Riccarton, Edinburgh, UK EH14 4AS. |
| This journal is © The Royal Society of Chemistry 2000 |
