Monitoring the ambient environment with diffusive samplers: theory and practical considerations†‡
Richard H.
Brown
Health and Safety Laboratory, Broad Lane, Sheffield, UK S3 7HQ
First published on 28th January 2000
1 Introduction
A diffusive sampler is a device which is capable of taking samples of gases or vapours from the atmosphere at a rate controlled by a physical process such as gaseous diffusion through a static air layer or a porous material and/or permeation through a membrane, but which does not involve the active movement of air through the device.1Such samplers offer significant advantages over those which do not involve the active movement of air. The most obvious one is that there is no pump or air mover to buy, maintain or calibrate. In addition, the sampler itself is usually very small and hence is lightweight and unobtrusive. As a consequence, in the occupational environment, it is more user-friendly, less inclined to influence worker behaviour and more amenable to self-assessment of worker exposure. In the ambient environment, it is easier to deploy and less susceptible to damage or theft. However, there are also disadvantages, the main one being a requirement to determine the effective sampling rate of the sampler itself (since the sampling rate is governed by the geometry of the sampler not an attached pump).
Diffusive sampling in the occupational environment dates back at least to the 1930s, when qualitative devices were described, but the first serious attempt to apply science to quantitative diffusive sampling was in 1973, when Palmes and Gunnison described a tube-form diffusive sampler for sulfur dioxide.2 Since then, a wide variety of diffusive samplers have been described, some relying on diffusion through an air gap, some relying on permeation through a membrane and some using both techniques.3–6 Many of these devices are commercially available. Industrial applications have included a wide variety of gases and vapours, both organic and inorganic.6,7
The use of diffusive sampling for monitoring the non-industrial environment, however, is relatively recent. In 1993, the International Union of Pure and Applied Chemistry (IUPAC) published a review of the potential of diffusive sampling for monitoring ambient air quality8 and Wolkoff in 1995 reviewed the potential of diffusive sampling for monitoring indoor air quality in a more general review of measurement techniques9 with specific reference to volatile organic compounds.
The successful practical application of diffusive sampling to ambient air, however, requires an understanding of the operating principles of diffusive sampling and an examination of the environmental factors which may affect sampler performance. These factors, e.g., temperature and wind speed, may be much more variable and severe than for workplace applications, and it is usually necessary to take precautions to protect against such adverse conditions.
2 European Directives and standardisation
In addition to the European Union (EU) ‘framework' Directive on Ambient Air Quality Assessment and Management,10 several associated Daughter Directives have been promulgated or are under development. These Directives prescribe performance requirements as data quality objectives (DQOs), including measurement uncertainty, minimum data capture and minimum time coverage. DQOs are set at different levels for different pollutants and also for different assessment methods: mandatory measurement, indicative measurement, modelling or objective estimation. Usually, a ‘reference' method is prescribed, but other methods, meeting the DQOs, may be used.The task of developing appropriate standards for ambient air quality measurements within the European Community has been carried forward by working groups (WGs) of Technical Committee TC 264 of CEN (Comité Européen de Normalisation).
The primary task of CEN TC 264 is to evaluate and recommend reference methods where these are not already prescribed in Directives. However, in the specific case of diffusive samplers which are likely to be used for indicative measurement, performance requirements standards11,12 are being developed by CEN/TC 164/WG11. A guide to the selection, use and maintenance of diffusive samplers for ambient air applications is also being developed.13
3 Operating principles
3.1 Principles of diffusive sampling
Diffusive sampling relies on the principles of Fick's law, which can be expressed as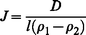 | (1) |
The mass of the analyte which can diffuse to a suitable sorbent within a certain time is determined by eqn. (2a), which is derived from eqn. (1):
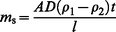 | (2a) |
Ideally, ρ1 is equal to the concentration of the given analyte in the air outside the diffusive sampler (ρ) and ρ2 equals zero (‘zero sink' condition). In that case, the magnitude of the diffusive uptake rate, AD/l [=U; see eqn. (3)], is dependent only on the diffusion coefficient of the given analyte and on the geometry of the diffusive sampler used.
In Fig. 1, the inlet of a sampler with cross-section D at position 1 defines the beginning of the diffusion path of an analyte with a concentration of ρ1. A sorbent at position 2, which will reduce the concentration of the analyte, ρ2, to zero (ideally) due to sorption or chemical reaction, serves as the driving force for the diffusion along l.
 | ||
| Fig. 1 Diagram of diffusion process. | ||
In practice, there are a number of factors that can give rise to non-ideal behaviour, so that
 | (2b) |
3.2 Dimensions of diffusive uptake rate
Rearranging eqn. (2b) and substituting U for AD/l: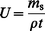 | (3a) |
Although the uptake rate, U, has dimensions of volume per unit time, this does not indicate a real volumetric flow of (analyte in) air. However, it may be convenient to define an ‘effective sampling volumetric flow rate', which is equivalent to that which would apply for an active sampler.
Diffusive uptake rates are very often quoted in units of pg ppb−1 min−1. These are practical units, since most environmental analysts use ppb for concentrations of gases and vapours [ppb is volume fraction (ϕ) = 10−9; ppm is volume fraction (ϕ) = 10−6]. The dependence of uptake rates on temperature and pressure is explained below. Thus, for a given concentration (ppb) of gas or vapour, the sampling rate is given by
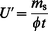 | (3b) |
Ideal and practical diffusive uptake rates are related by
 | (4) |
3.3 Bias due to the selection of a non-ideal sorbent
The performance of a diffusive sampler depends critically on the selection and use of a sorbent or collection medium which has a high sorption efficiency. The residual vapour pressure of the sampled compound at the sorbent surface (ρ2) will then be very small in comparison with the ambient concentration, and the observed uptake rate will be close to its ideal steady-state value, which can usually be calculated from the geometry of the sampler and the diffusion coefficient of the analyte in air.In the case where a weak sorbent is used, then ρ2 in eqn. (2a) is non-zero and ms/t will decrease with the time of sampling. In the alternative expression, eqn. (2b), k has a value significantly less than unity. Hence U in eqn. (3) will also decrease with the time of sampling. The concentration of the sampled pollutant can also have a (lesser) effect on ms/t and hence on U. The magnitude of these effects is dependent on the adsorption isotherm of the analyte and sorbent concerned, and may be calculated with the aid of computer models.14,15
Another manifestation of the same effect is back-diffusion, sometimes called reverse diffusion. This can happen where, some time after sampling has started, the vapour pressure of the analyte at the sorbent surface, ρ2, is greater than the external concentration, ρ1, for example if a sampler is first exposed to a high concentration and then to a much lower or even zero concentration. This type of exposure profile can occur in certain applications, and the magnitude of any error introduced will depend on whether the period of high concentration occurs at the beginning, middle or end of the sampling period. The phenomenon has been discussed in detail by Bartley et al.16–18 and a simple test proposed19 to give an estimate of the maximum bias to be expected between a pulsed exposure and an exposure to a constant concentration, which normally provides the basis for the sampler calibration. This test is 30 min exposure to high concentration, followed by 7.5 h of clean air, and has been adopted in EN 838.20 For ambient air applications,12 however, it is considered that an exposure profile of alternate periods of high and low exposure [e.g., 12 h at twice the relevant limit value (LV) followed by 12 h at zero concentration alternately for 7 d] is more typical of the intended application, where diurnal variations in concentrations are common. The extent of back-diffusion can also be modelled theoretically.15,21
It is therefore desirable to choose a sorbent with high sorption capacity and low vapour pressure of the sorbed material or of the reaction product formed by a reactive sorbent.
3.4 Environmental factors affecting sampler performance
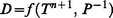 | (5) |
 | (6) |
 | (7) |
 | (8) |
3.4.4.1 Effect of low and high wind speeds. Ambient air movement and sampler orientation (relative to external air flow) can affect the performance of a diffusive sampler because they may influence the effective diffusion path length.4,28–30 The diffusive mass uptake of a sampler [eqn. (2)] is a function of the length, l, and the cross-sectional area, A, of the diffusion gap within the sampler. The nominal diffusion pathlength is defined by the geometry of the sampler and is the distance between the sorbent surface and the external face of the sampler. The cross-sectional area is also defined by the geometry of the sampler and if the cross-section of the diffusion gap is not constant along its length, is defined by the narrowest portion. The effective length, l, is not necessarily the same as the nominal length, and may be greater or less, depending on circumstances.
Under conditions of low external wind speeds, there may be insufficient air movement to replenish gas molecules close to the sampler surface that are being removed by diffusion. Under such conditions, the effective diffusion pathlength may be increased.29–30 This is because a ‘boundary layer'27,28 exists between the stagnant air within the sampler and the moving air outside and contributes to the effective diffusion pathlength, l. In reality, there is an area outside the sampler where there is a transition between static air and moving air, but this is equivalent to an extra length (l![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) ) of static air which must be included in the value of l. The value of l depends on the external geometry of the sampler, being roughly proportional to the linear cross-section of the sampler collection surface, where this surface is flat. It also decreases with increasing air velocity. Its significance depends on the value of the nominal path length of the diffusive sampler. Thus a sampler with a small cross-section and long internal air gap will be relatively unaffected by air velocity, whilst a short, fat sampler will be significantly affected. This is borne out in practice, as has been demonstrated with samplers of varying length.29,30 Low sampling rates are observed at low air velocities, but increase to a plateau value as the boundary layer effect becomes insignificant.
) of static air which must be included in the value of l. The value of l depends on the external geometry of the sampler, being roughly proportional to the linear cross-section of the sampler collection surface, where this surface is flat. It also decreases with increasing air velocity. Its significance depends on the value of the nominal path length of the diffusive sampler. Thus a sampler with a small cross-section and long internal air gap will be relatively unaffected by air velocity, whilst a short, fat sampler will be significantly affected. This is borne out in practice, as has been demonstrated with samplers of varying length.29,30 Low sampling rates are observed at low air velocities, but increase to a plateau value as the boundary layer effect becomes insignificant.
Under conditions of high external wind speeds, the effective diffusion pathlength may be decreased.31–37 This is because external high air flows disturb the static air layer within the sampler, which reduces the effective air gap by a (different) factor l. The value of l is small, provided that the length to diameter ratio of the sampler air gap is greater than 2.5–3,31 or it can be avoided, or greatly reduced, by incorporating a draught shield, e.g., a stainless steel screen or plastic membrane.
3.4.4.2 Turbulence and sampler orientation. These factors are less well understood. In practice, ambient air is likely to be turbulent, whereas for practical reasons most laboratory experiments are conducted under laminar flow conditions, where the (‘linear') air velocity can be more easily measured. It is to be expected that the experiments assessing the effects of air movement under such laboratory conditions will underestimate, relative to the situation in real ambient air, the points where the low and high wind speed effects become significant.
The (linear) air velocity is often referred to as ‘face velocity'. This does not necessarily imply that the air movement is across the sampling surface of the sampler, but this is often the case in the laboratory experiments referred to in the previous paragraph. Differences may be observed in the sampling rate for samplers parallel and perpendicular to the air flow, especially for open-ended samplers, but under (turbulent) ambient air conditions, the difference is likely to be small.
3.4.4.3 Consequence for different sampler geometries. Tube-type samplers are typically unaffected by low air velocities,22,38,39 but those without a draught shield may be affected by high speeds.
Badge-type samplers generally have a large surface area and small air gap, so that they may be more affected by air velocity than tube designs and typically require a minimum face velocity40–43 of between 0.5 and 0.2 m s−1. Some badges with an inadequate draught shield are also affected at high air velocities.39,41,44
Radial diffusive samplers45,46 require a minimum face velocity of about 0.25 m s−1.
4 Transportation
Most samplers will require transportation between the sampling site and an analytical laboratory, so that it is important that sample integrity is maintained during this process. The following precautions are recommended.(a) Ensure that any seals are sufficiently tight to avoid ingress of contamination or loss of sample during transit: metal–plastic seals may become loose if a large temperature change takes place.
(b) Place the samplers in inert closed containers to minimise the ingress of external contamination.
(c) If air-freighting samples, ensure that they are not subjected to negative pressure, e.g., in the baggage hold.
(d) Avoid exposure to high temperatures during transit, e.g., in the boot of a car.
(e) If possible, keep the samplers at low temperatures and away from contamination sources, e.g., petroleum or aviation fuel, but avoiding condensation on the sample.
Ensure that adequate sample blanks are transported with the samples so that any of the above problems can be identified.
5 Protection from adverse environmental conditions
In practical use in the ambient environment, attention has to be paid to three main considerations: air velocity, protection from precipitation and security.5.1 Air velocity
Monthly averages of ambient wind velocities in Europe47 are mostly in the range 1–10 m s−1 but can fall below 0.5 m −1 temporarily in the case of stable meteorological conditions (inversions) and/or locally in valleys of mountainous areas.48,49 Moreover, at least for local source emissions, pollutant concentrations are inversely proportional to wind speed47 so any sampling error at low wind speeds would be magnified in the time-weighted average.For samplers which are affected at low wind speeds, and if exposure to such conditions is likely to be significant, then some additional ventilation is necessary. This could be achieved by using a small fan for forced ventilation: this might defeat the object of using a ‘passive' sampler, but could be practicable with solar power in more southern climates. Alternatively samplers might be suspended on a fine thread to increase the effect of small air movements.8
Similarly, for samplers which are affected at high wind speeds, some attenuation of the air velocity is necessary.
Even where moderate wind speeds are expected, problems can arise if samplers are placed too close to buildings or other obstacles. Consideration should be given to the size and location of the obstacle(s) in the siting of the sampler.
5.2 Water
Protection from precipitation is important for all types of sampler. Rain or melted snow can block the sampling surfaces,50 particularly of tube samplers oriented vertically downwards (which is the usual position to avoid ingress of particulates).5.3 Provision of a shelter
In order to minimise the potential problems identified above, a shelter should be used when exposing susceptible diffusive samplers. The shelter has to be optimised for the sampler type, taking the following into account:(a) The shelter should protect the sampler from high air velocities and precipitation, but there should be sufficient air movement for the air inside the shelter to be representative of the air outside and to satisfy any minimum velocity requirement;
(b) The design of the shelter and the means of locating the sampler should not significantly affect the diffusive uptake rate of the sampler or the wind direction;
(c) The construction, surface and colour of the shelter should be such as to minimise thermal gain in sunny periods.
A simple shelter for a tube-type sampler may consist of an inverted plastic funnel located such that the tube fits snugly into the spout of the funnel (cut short if necessary) and the open end of the tube is just visible below the funnel opening.51 This example is illustrated in Fig. 2. Another possibility is an inert ‘nest box', with holes drilled in the bottom. The tube(s) are placed inside the box, again with the open end of the tube just visible below the opening.
 | ||
| Fig. 2 Sampling tube with protective weather hood. Note that other samplers and alternative protection devices to the funnel shown may be employed. | ||
Badge-type diffusive samplers could also be located in a box, such as a Stevenson screen. It is necessary to have holes or slats in the side(s) of the box to allow sufficient air movement. Various ad hoc methods have been used, e.g., an angled aluminium plate.52
If the primary objective is to avoid high wind-speed effects, an inert shelter such as is illustrated in refs. 53–55 should be used to restrict high winds suitably whilst maintaining the required minimum air velocity where possible.
An alternative to providing a shelter is to modify the diffusive sampler. For example, the diffusion end-cap of a tube-type sampler can be modified with the addition of an aluminium brim to prevent rainwater blocking the diffusion surface.56 However, such an arrangement may modify the performance characteristics of the sampler with respect to the minimum air velocity requirement.
5.4 Security
Security is also a major consideration, as samplers exposed for long periods in public or semi-public places are subject to theft and vandalism. Samplers should be sited, as far as possible, to be out of reach, inconspicuous and/or designed to look like something else, e.g., the nest box idea.6 Arrangement of sampling points
The number and location of sampling points should be selected in such a way, as a function of the measurement task, that answers to the specific questions posed can reasonably be expected. Once chosen, the sampling points should not be changed during the measurement programme.Unless specified otherwise, suitable sampling points should be at least 1 m away from buildings or other major obstructions to avoid local perturbation effects. The measuring height should be between 1.5 and 4 m: at least 2.5 m will discourage theft and vandalism. The immediate vicinity of trees, bushes, etc., should be avoided to minimise effects from troublesome insects.
7 Requirements for training
For most apparatus, specialist training is required for both the operator and those responsible for the maintenance and calibration. Diffusive samplers, however, do not require a specialist operator for their deployment, provided that clear and unambiguous instructions for use are available and due note is taken of the above guidance. A minimum of practical training is indispensable for operators to avoid common errors such as smoking during work, working directly near a car with its engine running, touching inner surfaces of the sampler with the fingers or using felt-tip pens for marking.Maintenance and calibration, however, are as important for diffusive samplers as for other devices, and particular attention should be made to the following:
(a) For re-usable devices, ensure that the collection element is intact, clean or replaced as necessary;
(b) Ensure that all component parts are free from contamination;
(c) Ensure that the devices are calibrated for typical exposure regimes of concentration, time of exposure, humidity and wind velocity;
(d) Ensure that devices are used within the manufacturer's recommended shelf-life.
8 Quality assurance (QA)
It is good practice to set up a quality assurance scheme for the maintenance and calibration of the samplers. This includes:(a) The establishment of a standard operating procedure (SOP);
(b) For re-usable devices, a log of usage;
(c) Keeping a record of the traceability of the calibration;
(d) Retaining the raw data as required by the quality or other system;
(e) Using a unique and durable sampler numbering system for re-usable devices;
(f) Depending on the measurement task, taking an appropriate number of field blank and replicate samples (e.g., 10%);
(g) For internal QA, to check diffusive sampling rates routinely, at least once during large surveys; this may be done by exposing samplers in laboratory standard atmospheres or by laboratory or field comparisons with an independent (e.g., pumped) method;
(h) For external QA, to check diffusive sampling rates routinely, at least once during large surveys; this may be done by laboratory or field intercomparisons, which should be conducted by experienced institutes.
9 Design of standard atmosphere apparatus
The calibration of a diffusive sampler uptake rate requires the construction and use of a dynamic system for generating, pre-mixing and delivering a known concentration of a test gas or vapour.57–65Additional practical guidance in setting up a syringe injection system is given in an HSE publication66 and for a permeation tube system in the HSE publication.67 An evaluation of the performance of the syringe injection system is given in reference 68, which also gives an example of a suitable sampling manifold or exposure chamber for tube-type diffusive samplers. An improved design, incorporating facilities for temperature and humidity control, was described in Pengelly et al.69 This includes a second design of exposure chamber, incorporating a stirrer motor to generate sufficient air movement for badge-type samplers. A description of a capillary dosage system was given in Goelen et al.70
10 Conclusion
The application of the above guidance should result in the successful application of diffusive sampling to appropriate areas in environmental air quality assessment. Some practical examples are given in Brown8 and have been updated in ‘Specific applications' below. A prerequisite is, of course, to use a diffusive sampler that has been validated in laboratory and field experiments according to an established protocol, such as the CEN draft standards.11–12 However, it will still be necessary to apply the above principles intelligently and to investigate fully any environmental conditions experienced in the field application that are not covered by the tests used in the validation.11 Specific applications
This section gives some examples of practical applications of diffusive samplers for the monitoring of ambient air. It is intended as a guide to sources of information and should not replace the evaluation of the suitability of a particular device for a particular application as described above. The source references should be consulted before operational use of the devices.The following information, whilst intended to be as comprehensive as possible, of necessity reflects only part of a rapidly increasing literature. Important new developments may therefore be missing or only partly described. The main text is restricted to a brief description of the main devices available, important advantages or limitations and validation status. In most cases, a full validation according to Parts 1 and 2 of the draft CEN Standard11,12 has not been conducted (since the Standard was not available at the time) and this should be borne in mind when selecting suitable devices.
11.1 Nitrogen oxides
The Palmes tube is very simple and cheap, but in the open-tube version is susceptible to high wind speeds.72 It may also have blank problems71,73,74 and peroxyacetyl nitrate may be a positive interferent.75
Many workers have shown good agreement with alternative measurement methods in field intercomparisons.33,74,76,77
Such samplers have higher intrinsic sampling rates than tube types, so exposure periods can be shorter. Since the chemistry is the same, chemical effects and interferences will be similar. Moschandreas et al.84 found no effect of temperature or humidity for the Mulik badge, except at extremes of temperature. However, Krochmal and Kalina85 found a significant temperature effect for NO2 (but not for SO2).
Many workers have shown good agreement with alternative methods in field intercomparisons.84–87
11.2 Nitrogen monoxide
In principle, the Palmes tube can also be used to monitor NO if oxidised to NO2. Indeed, an early paper by Palmes and Tomczyk88 refers to an NOx sampler which monitors both NO and NO2. Sampled NO (together with any NO2) diffuses down the tube, through the TEA-coated screen, where it meets an oxidising layer and diffuses back as NO2. NO is measured by difference between tubes with and without oxidising layer. In the Palmes version, the oxidising layer is a chromic acid-coated screen which has to be inserted immediately before sampling and removed immediately afterwards. The same principle is used in a badge design by Ferber et al.89 (which incidentally predates the EPA/Monsanto badge).A further device that can be used for both NO and NOx is marketed by Ogawa. The technology was developed by Hirano in Yokohama, Japan, but has not been published. It is a dual-faced sampler in which NO2 is determined on one side and NOx on the other; NO is determined by difference. TEA is used to collect NO2 and a proprietary reagent for NOx. A joint study by the USEPA, the Harvard School of Public Health and ManTech Environmental Technology90 demonstrated good agreement between Ogawa PSDs and weekly real-time averages from instrumental methods.
11.3 Sulfur dioxide
As noted above, the Palmes tube can also be used for monitoring sulfur dioxide. Triethanolamine forms a complex with SO2 in addition to NO2 and the product may be analysed by the p-rosaniline method or by ion chromatography after elution of TEA with a hydrogen-carbonate buffer. Preliminary results76 suggest a high correlation with a fluorescence monitor under field conditions, but the response was only 50% of the expected value. More recently,91 problems were reported with storage recovery and the effect of relative humidity. These problems have now been largely overcome.92,93Similarly, several badge designs have been described. Killick94 evaluated the West badge95 for ambient SO2 measurement in laboratory tests, sampling over 1–19 d. He found generally good agreement with a continuous conductiometric instrument: in 75% of tests, the ratio of badge to instrument results was between 0.80 and 1.17. Orr et al.96 modified the West badge to incorporate a porous (diffusion-limited) membrane rather than a silicone membrane and a potassium carbonate–glycerol-impregnated filter rather than an absorption solution. The modified badge had a higher collection rate but also greater dependence on air velocity. Also to gain higher sensitivity, Scheeren et al.97 used a short circular badge to a design by Willems and Hofschreuder83 and investigated both TEA and potassium carbonate as collection media. De Santis et al. developed this further to a two-badge design.82 Similar devices have been described by Kaspar-Giebl and co-workers.81,98
Hallberg and Rudling99 designed a liquid medium sorbent badge, in which the diffusion is controlled by a number of small channels. The sampler was designed to be versatile; sodium carbonate–hydrogencarbonate buffer was used as the sorbing medium for SO2, and dilute sulfuric acid was used as the sorbing medium for ammonia (see below).
11.4 Ammonia
Frenzel et al.37,100 also used the Palmes tube principle successfully for NH3. He uses standard laboratory glass vials (4.3 × 1.72 cm diameter), the bottom of which have been treated with phosphoric acid. Ammonia (as ammonium phosphate) is dissolved in 50 µl of water and determined by flow injection analysis. The detection limit was 1.2 µg m−3 for a sampling time of 24 h. Field trials against impinger sampling were encouraging, but were complicated by the difficulty of eliminating particulate matter in the impingers by pre-filters.In the range of badge-type samplers, Willems and Hofschreuder83 described an extension of the Benedict sampler. As with the EPA/Monsanto badge for NO2, the modification maximises the sampling rate by having a design of large area and short diffusion path, and relies on an impregnated filter for adsorption. Tartaric acid was found to be the best coating. Excellent correlation between monitors and an impinger reference method was found in field trials; a small bias (about 10%) was noted, but not explained. Concentrations of (particulate) ammonium salts in The Netherlands were not thought sufficient to explain the higher impinger results. More recent studies have included those by Ferm and Rohde101 and Kasper and Puxbaum.43
11.5 Organic gases (volatile organic compounds)
One version of the tube design22 uses a thermally desorbable sorbent, and the diffusion path is simply an air gap at one end of the tube between the sorbent and a draught screen. The tube was designed originally for workplace monitoring102 but its use has been extended to ambient air.103–107 Very high sensitivity can be achieved with thermal desorption, but there is a disadvantage from the application of thermally reversible sorption, which means that the sampling rate may not be constant108 because of back-diffusion.21 For volatile compounds such as benzene, a different calibration must be used for the time of exposure (1, 2 or 4 weeks) and a different rate applied to ambient and workplace concentration levels.107,109 The use of a double layer of sorbent may result in more constant sampling rates.110 A second problem with thermal desorption methods is that some sorbents, particularly Tenax, give rise to artefacts on reaction of the polymeric sorbent with NOx and ozone. The levels are very low and are insignificant for workplace air but may be serious in environmental applications.111 Conditions for minimising artefact formation were given by Helmig.112 The diffusive sampling results were compared with those of continuous monitoring methods based on gas chromatography and show generally good agreement between the two methods.109,113 In other studies,21,107,114 the continuous monitoring method as been assumed to give the ‘true value' and the comparison used to calibrate the diffusive sampler uptake rate. A summary of practical environmental diffusive sampling rates for benzene, toluene and xylene was given by Brown.115
A second version of the tube design uses charcoal and solvent desorption. Also originally designed for workplace air monitoring, it has been used successfully for sampling ambient air.21,116,117 Because charcoal has a different sorption mechanism, significant back-diffusion is not observed for benzene, toluene and xylene.21 For ambient air monitoring, samples are exposed over periods of 2 or 4 weeks. The results have been compared with those of continuous monitoring methods based on gas chromatography and show generally good agreement.21,118–120
A charcoal-based badge sampler has also been used for both ambient and indoor air monitoring.126 However, studies with three commercial badges126–128 found that generally high blanks and poor sensitivity limited their usefulness for ambient air monitoring.
11.6 Formaldehyde
One of the most rapid and sensitive methods of determining formaldehyde is HPLC of the 2,4-dinitrophenylhydrazone (DNPH) of formaldehyde. This method has been used in workplace air monitoring as a pumped DNPH-coated filter method and can give a sensitivity of about 1 µg m−3 (1 ppb) for a 50 l sample.130 These authors also developed a diffusive version131 and demonstrated that it could be used to monitor down to 5 ppb in an 8 h sample. An almost identical diffusive sample has been developed by Grosjean and Williams.132The GMD formaldehyde badge is also based on the reaction of DNPH on an impregnated filter. The sensitivity is about 3 ppb for a 24 h sample.133
The EPA/Monsanto badge has also been modified for the measurement of formaldehyde.78 However, the EPA does not currently advocate its use because of blank problems (R. Lewis, personal communication). Ishii and Aoki134 have described a diffusion sampler based on DNPH which uses an absorption solution of tetramethylene sulfone–water–DNPH. The authors claim a lower detection limit of 2 ppb for 24 h sampling.
Artefact problems may arise when using DNPH-based samplers in atmospheres containing relatively high ozone levels,135 although this has been disputed by J. O. Levin (unpublished work). Grosjean and Williams132 found that spiked filters mounted in diffusive samplers and exposed to 120 ppb of ozone for 24 h lost approximately 56% of the hydrazone, but admitted that this exposure to ozone was unrepresentative of most outdoor situations. Stability of the hydrazone may be a problem with adsorption tubes (H. Oehme, personal communication), although an extensive study undertaken on behalf of the BCR136,137 suggests that both impregnated filters and solutions are stable for many months.
A Palmes-type tube has also been used for formaldehyde, e.g., as described by Hangartner138 and Preschler and Schöndube.139
11.7 Ozone
Hangartner138 used a Palmes-type tube with a dipyridylethylene adsorption medium and 3-methyl-2-benzothiazolinone hydrazone (MBTH) analysis of the released aldehyde. Similar systems have been studied by Kirchner et al.140 and Striedner.141A colorimetric diffusive sampler has been developed for ozone,142 which is based on a colorant (indigo carmine) which fades on reaction with ozone and can be estimated by reflectance colorimetry. Field tests have been conducted by Grosjean and Williams143 and show little interference from NOx, peroxyacetyl nitrate or formaldehyde. Similar systems have been studied by Werner,144 Hangartner et al.145 and Monn and Hangartner.146
The Ogawa sampler has also been used for ozone147 by making use of the oxidation of nitrite to nitrite by ozone.
References
- Diffusive Sampling, an Alternative Approach to Workplace Air Monitoring, ed. A. Berlin, R. H. Brown and K. J. Saunders, EC Publication No. 10555 EN, EC, Brussels, Luxembourg, 1987. Search PubMed.
- E. D. Palmes and A. F. Gunnison, Am. Ind. Hyg. Assoc. J., 1973, 34, 78 CAS.
- W. Jost, Diffusion in Solids, Liquids and Gases, Academic Press, New York, 1960, pp. 42–45. Search PubMed.
- F. C. Tompkins and R. L. Goldsmith, Am. Ind. Hyg. Assoc. J., 1977, 38, 371 CAS.
- R. L. Bamberger, G. G. Esposito, B. W. Jacobs, G. E. Podolak and J. F. Mazur, Am. Ind. Hyg. Assoc. J., 1978, 39, 701 CAS.
- R. H. Brown, in Clean Air at Work, ed. R. H. Brown, M. Curtis, K. J. Saunders and S. Vandendriessche, EC Publication No. EUR 14214, EC, Brussels, Luxembourg, 1992, pp. 142–148. Search PubMed.
- D. C. M. Squirrell, in Diffusive Sampling, ed. A. Berlin, R. H. Brown and K. J. Saunders, CEC Publication No. 10555 EN, Royal Society of Chemistry, London, 1987, pp. 29–45. Search PubMed.
- R. H. Brown, Pure Appl. Chem., 1993, 65, 1859 CrossRef CAS.
- P. Wolkoff, Indoor Air, 1995, Suppl. 3/95. Search PubMed.
- Council Directive 96/62/EC on Ambient Air Quality Assessment and Management, EU, Brussels, 1996..
- Comité Européen de Normalisation, Ambient Air Quality – Diffusive Samplers for the Determination of Concentrations of Gases or Vapours – Part 1: General Requirements, prEN 13528-1, CEN, Brussels, 1999. Search PubMed.
- Comité Européen de Normalisation, Ambient Air Quality – Diffusive Samplers for the Determination of Concentrations of Gases or Vapours – Part 2: Specific Requirements and Test Methods, prEN 13528-2, CEN, Brussels, 1999. Search PubMed.
- Comité Européen de Normalisation, Ambient Air Quality – Diffusive Samplers for the Determination of Concentrations of Gases or Vapours – Part 3: Guide for Selection, Use and Maintenance, prEN 13528-3, CEN, Brussels, 1999. Search PubMed.
- N. Van den Hoed and O. L. J. van Asselen, Ann. Occup. Hyg., 1991, 35, 273 CrossRef CAS.
- E. Nordstrand and J. Kristensson, Am. Ind. Hyg. Assoc. J., 1994, 55, 935 CAS.
- D. L. Bartley, L. J. Doemeny and D. G. Taylor, Am. Ind. Hyg. Assoc. J., 1983, 44, 241 CrossRef CAS.
- D. L. Bartley, Am. Ind. Hyg. Assoc. J., 1983, 44, 879 CrossRef CAS.
- D. L. Bartley, M. L. Woebkenberg and J. C. Posner, Ann. Occup. Hyg., 1988, 32, 333 CAS.
- D. L. Bartley, G. J. Deye and M. L. Woebkenberg, Appl. Ind. Hyg., 1987, 2, 119 Search PubMed.
- Comité Européen de Normalisation, Workplace Atmospheres – Requirements and Test Methods for Diffusive Samplers for the Determination of Gases and Vapours, EN 838, 1995. Search PubMed.
- J. S. Posner and G. Moore, Am. Ind. Hyg. Assoc. J., 1985, 46, 277 CrossRef CAS.
- H.-U. Pfeffer L. Breuer K. Ellermann, Validierung von Passivsammlern für Immissionsmessungen von Kohlenwasserstoffen, Materialien Nr. 46, Landesumweltamt NRW, Essen, 1998. Search PubMed.
- R. H. Brown, J. Charlton and K. J. Saunders, Am. Ind. Hyg. Assoc. J., 1981, 42, 865 CAS.
- F. J. Hearl and M. P. Manning, Am. Ind. Hyg. Assoc. J., 1980, 41, 778 CAS.
- D. W. Underhill, Am. Ind. Hyg. Assoc. J., 1983, 44, 237 CrossRef CAS.
- H. Hori and I. Tanaka, Am. Ind. Hyg. Assoc. J., 1993, 54, 95 CrossRef CAS.
- J. R. Compton, G. S. Dwiggins, C. E. Feighley and D. A. Ludwig, Am. Ind. Hyg. Assoc. J., 1984, 45, 446 CrossRef CAS.
- D. W. Underhill and C. E. Feighley, Anal. Chem., 1991, 63, 1011 CrossRef CAS.
- L. Pozzoli D. Cottica, in Diffusive Sampling, ed. A. Berlin, R. H. Brown and K. J. Saunders, CEC Publication No. 10555 EN, Royal Society of Chemistry, London, 1987, pp. 119–130. Search PubMed.
- N. Zurlo F. Andreoletti, in Diffusive Sampling, ed. A. Berlin, R. H. Brown and K. J. Saunders, CEC Publication No. 10555 EN, Royal Society of Chemistry, London, 1987, pp. 174–176. Search PubMed.
- S. R. Coleman, Am. Ind. Hyg. Assoc. J., 1983, 44, 929 CrossRef.
- E. D. Palmes, A. F. Gunnison, J. DiMatto and C. Tomczyk, Am. Ind. Hyg. Assoc. J., 1976, 37, 570 CAS.
- D. H. F. Atkins J. Sandalls D. V. Lawland A. M. Hough, The Measurement of Nitrogen Dioxide in the Outdoor Environment Using Passive Diffusion Tube Samplers, AERE-R12133, AERE, Harwell, 1986. Search PubMed.
- A. J. Gair and S. A. Penkett, Atmos. Environ., 1995, 29, 2529 CrossRef CAS.
- C. E. C. Downing G. W. Campbell J. C. Bailey, A Survey of Sulphur Dioxide, Ammonia and Hydrocarbon Concentrations in the United Kingdom Using Diffusion Tubes: July to December 1992, Report LR 964, Warren Spring Laboratory, Stevenage, 1994. Search PubMed.
- M. Fern, A Sensitive Diffusional Sampler, Report B1020, Institutet for Vatten och Luftvardsforskning, Gothenberg, 1991. Search PubMed.
- W. Frenzel, E. Grimm and G. Gruetzmacher, Fresenius' J. Anal. Chem., 1995, 351, 19 CrossRef CAS.
- K. H. Pannwitz, in Diffusive Sampling, ed. A. Berlin, R. H. Brown and K. J. Saunders, CEC Publication No. 10555 EN, Royal Society of Chemistry, London, 1987, pp. 157–160. Search PubMed.
- D. Mark A. Robertson H. Gibson G. Borzucki B. Cherrie W. M. Maclaren, Evaluation of Diffusive Samplers for Monitoring Toxic Gases and Vapours in Coal Mines, Report TM/90/11, Institute of Occupational Medicine, Edinburgh, 1990. Search PubMed.
- M. Harper and C. J. Purnell, Am. Ind. Hyg. Assoc. J., 1987, 48, 214 CrossRef CAS.
- H. Hori and I. Tanaka, Ann. Occup. Hyg., 1996, 40, 467 CrossRef CAS.
- R. G. Lewis, J. D. Mulik, R. W. Coutant, G. W. Wooten and C. R. McMillin, Anal. Chem., 1985, 57, 214 CrossRef CAS.
- A. Kasper and H. Puxbaum, Fresenius' J. Anal. Chem., 1994, 350, 448 CrossRef CAS.
- B. S. Samini, in Diffusive Sampling, ed. A. Berlin, R. H. Brown and K. J. Saunders, CEC Publication No. 10555 EN, Royal Society of Chemistry, London, 1987, pp. 166–169. Search PubMed.
- V. Cocheo, C. Boaretto and P. Sacco, Am. Ind. Hyg. Assoc. J., 1996, 57, 897 CrossRef CAS.
- P. Perez-Ballesta ERLAP, JRC, Ispra, Italy, personal communication..
- N. T. Plant M. D. Wright, European Diffusive Sampling Initiative: Pilot Surveys of Sheffield, Report IACS/96/1, Health and Safety Laboratory, Sheffield, 1996 (obtainable from the Health and Safety Laboratory, Broad Lane, Sheffield, UK S3 7HQ). Search PubMed.
- I. Troen E. L. Petersen, European Windatlas, Riso National Laboratory, Roskilde, 1990. Search PubMed.
- J. Christoffer M. Ulbricht-Eissing, Die Bodennahen Windverhältnisse in der BR Deutchland, Berichte des Deutchen Wetterdienstes, 147, Offenbach-a-M, 1989. Search PubMed.
- T. L. Hafkenschied and J. Mowrer, Analyst, 1996, 121, 1249 RSC.
- N. T. Plant M. D. Wright, European Diffusive Sampling Initiative: UK National Survey of BTX, Report IACS/97/6, Health and Safety Laboratory, Sheffield, 1997 (obtainable from the Health and Safety Laboratory, Broad Lane, Sheffield, UK S3 7HQ). Search PubMed.
- A. Hagenbjörk-Gustafsson, B. Forsberg, G. Hestvik, D. Karlsson, S. Wahlberg and T. Sandström, Analyst, 1996, 121, 1261 RSC.
- P. Rempler and W. Kosmus, Fresenius' J. Anal. Chem., 1988, 329, 871.
- VDI 2119, Part 4, Measurement of Particulate Precipitations: Microscopic Differentiation and Size Fractioned Determination of Particle Deposition on Adhesive Collection Plates: Sigma-2 Sampler, VDI, Verlag, Dusseldorf, Germany, 1997. Search PubMed.
- R. Eltschka J. Kühr E. Schultz, Luftverunreinigungen und Atemwegserkrankungen: Lufthygienische Messungen und Epidemiologische Untersuchungen im Raum Freiburg, Ecomed, 1994. Search PubMed.
- J. Mowrer, A. Potter and A. Lindberg, Analyst, 1996, 121, 1295 RSC.
- G. O. Nelson, Gas Mixtures: Preparation and Control, Lewis, Chelsea, MI, 1992. Search PubMed.
- VDI 3490, Parts 1–17, Calibration Gas Mixtures, Preparation of Calibration Gas Mixtures by..., Beuth, Berlin. Search PubMed.
- International Standards Organization, Gas Analysis – Calibration Gas Mixtures – Certificate of Mixture Preparations, ISO 6141, ISO, Geneva, 1984. Search PubMed.
- International Standards Organization, Gas Analysis – Preparation of Calibration Gas Mixtures – Weighing Methods, ISO 6142, ISO, Geneva, 1981. Search PubMed.
- International Standards Organization, Gas Analysis – Determination of Composition of Calibration Gas Mixtures – Comparison Methods, ISO 6143, ISO, Geneva, 1981. Search PubMed.
- International Standards Organization, Gas Analysis – Preparation of Calibration Gas Mixtures – Static Volumetric Methods, ISO 6144, ISO, Geneva, 1981. Search PubMed.
- International Standards Organization, Gas Analysis – Preparation of Calibration Gas Mixtures – Dynamic volumetric methods, ISO 6145, ISO, Geneva, 1986. Search PubMed.
- International Standards Organization, Gas Analysis – Preparation of Calibration Gas Mixtures – Permeation Method, ISO 6349, ISO, Geneva, 1979. Search PubMed.
- P. Jauen, N. Gonzalez-Flesca and P. Carlier, Environ. Sci. Technol., 1995, 29, 1718.
- Health and Safety Executive, Methods for the Determination of Hazardous Substances. Generation of Test Atmospheres of Organic Vapours by the Syringe Injection Technique. Portable Apparatus for Laboratory and Field Use, MDHS 3, HM Stationery Office, London, 1990. Search PubMed.
- Health and Safety Executive, Methods for the Determination of Hazardous Substances. Generation of Test Atmospheres of Organic Vapours by the Permeation Tube Method. Apparatus for Laboratory Use, MDHS 4, HM Stationery Office, London, 1986. Search PubMed.
- M. D. Wright, Generation of Standard Atmospheres by Syringe Injection – Design and Performance of a Compact System, Report IR/L/AO/83/15, Health and Safety Laboratory, Sheffield, 1983 (obtainable from the Health and Safety Laboratory, Broad Lane, Sheffield, UK S3 7HQ). Search PubMed.
- M. I. Pengelly P. A. Ellwood J. A. Groves, Generation of Test Atmospheres Using an Improved Design of Standard Atmosphere Apparatus, Report IR/L/SP/88/06, Health and Safety Laboratory, Sheffield, 1988 (obtainable from the Health and Safety Laboratory, Broad Lane, Sheffield, UK S3 7HQ). Search PubMed.
- E. Goelen, M. Lambrechts, F. Geyskens and T. Rymen, Int. J. Environ. Anal. Chem., 1992, 47, 217 Search PubMed.
- D. P. Miller, Atmos. Environ., 1987, 22, 945.
- D. H. F. Atkins C. Healy J. B. Tarrant, The Use of Simple Diffusion Tubes for the Measurement of NO2 Levels in Homes Using Gas and Electricity for Cooking, AERE Report R9184, AERE, Harwell, 1978. Search PubMed.
- J. J. Colls, Atmos. Environ., 1986, 20, 239 CrossRef CAS.
- A. J. Gair, S. A. Penkett and P. A. Oyola, Atmos. Environ., 1991, 25A, 1929.
- M. W. M. Hisham and D. Grosjean, Atmos. Environ., 1990, 24A, 2523 CAS.
- M. Hangartner P. Burri, in Diffusive Sampling, ed. A Berlin, R. H. Brown and K. J. Saunders, CEC Publication 10555 EN, Royal Society of Chemistry, London, 1987, pp. 387–391. Search PubMed.
- Stanger Science and Environment, Validation of Nitrogen Dioxide Diffusion Tube Methodology, draft 26.5.98, NETCEN, Abingdon, Oxfordshire, UK, 1998. Search PubMed.
- J. D. Mulik, R. G. Lewis, W. A. McClenny and D. D. Williams, Anal. Chem., 1989, 61, 187 CrossRef CAS.
- D. Krochmal and L. Gorski, Environ. Sci. Technol., 1991, 25, 531 CAS.
- Polish Standard PN/Z-04092/08, Wydawnictwo Normalizacyjne ‘Alpha', Warsaw, 1989..
- A. Kasper-Giebl Y. Garmroudi P. Biebl, Int. J. Environ. Anal. Chem., submitted for publication. Search PubMed.
- F. De Santis, I. Allegrini, M. C. Fazio, D. Pasella and R. Piredda, Anal. Chim. Acta, 1997, 346, 127 CrossRef CAS.
- J. J. H. Willems P. Hofschreuder, in Proceedings of a Field Study Organised by CEC and CNR – Institute for Atmospheric Pollution, Rome, ed. I. Allegrini, A. Febo and C. Perrino, Air Pollution Research Report 37, Guyot Brussels, 1990, pp. 113–121. Search PubMed.
- D. J. Moschandreas, S. M. Relwani, K. C. Taylor and J. D. Mulik, Atmos. Environ., 1990, 24A, 2807 CAS.
- D. Krochmal and A. Kalina, Atmos. Environ., 1997, 20, 3473 CrossRef.
- M. Hangartner, presented at World Congress on Air Pollution in Developing Countries, Costa Rica, October, 1996..
- Y. Yanagisawa and H. Nishimura, Environ. Int., 1982, 8, 235 CrossRef CAS.
- E. D. Palmes C. Tomczyk, Personal Sampler for NOx, Report, US Bureau of Mines, Washington, DC, 1977. Search PubMed.
- B. I. Ferber, F. A. Sharp and R. W. Freedman, Am. Ind. Hyg. Assoc. J., 1976, 37, 32 CAS.
- J. D. Mulik J. L. Varns P. Koutrakis M. Wolfson A. Bunyaviroch D. D. Williams K. G. Kronmiller, in Proceedings of the 1991 EPA/A and WMA International Symposium, Measurement of Toxic and Related Air Pollutants, Durham, NC, May 1991. Search PubMed.
- M. Hangartner P. Burri C. Monn, in Proceedings of the 8th World Clean Air Congress, The Hague, The Netherlands, September 1989, ed. L. J. Brasser and W. C. Mulder, Elsevier, Amsterdam, 1989, vol. 3. Search PubMed.
- M. Ferm P. A. Svanberg, Atmos. Environ., in the press. Search PubMed.
- U. Voltti S. Juntto, in Proceedings of the 10th World Clean Air Congress, Espoo, Finland, 1995, vol. 2, pp. 354–355. Search PubMed.
- C. M. Killick, Environ. Sci. Technol., 1976, 10, 473 CAS.
- K. D. Reiszner and P. W. West, Environ. Sci. Technol., 1973, 7, 523.
- D. B. M. Orr, J. C. Hiffner, W. H. Chan, M. A. Lusis and J. E. Hunt, Atmos. Environ., 1987, 21, 1473 CAS.
- B. A. Scheeren, F. De Santis, I. Allegrini and P. Heeres, Int. J. Environ. Anal. Chem., 1994, 56, 72 Search PubMed.
- A. Kaspre-Giebl S. Krenn H. Puxbaum, Fresenius' J. Anal. Chem., submitted for publication. Search PubMed.
- B. O. Hallbreg and J. Rudling, Ann. Occup. Hyg., 1989, 33, 61.
- W. Frenzel, VDI Ber., 1996, 1256, 333 Search PubMed.
- M. Ferm and H. Rodhe, J. Atmos. Chem., 1997, 27, 17 CrossRef CAS.
- Health and Safety Executive, Methods for the Determination of Hazardous Substances. Volatile Organic Compounds in Air. Laboratory Method Using Diffusive Solid Sorbent Tubes, Thermal Desorption and Gas Chromatography, MDHS 80, HM Stationery Office, London, 1995. Search PubMed.
- R. H. Brown, Pure Appl. Chem., 1995, 67, 1423 CAS.
- E. De Saeger and P. P. Ballesta, Diffus. Monit., 1995, 7, 7 Search PubMed.
- R. Peters and Th. Hafkenscheid, Diffus. Monit., 1995, 7, 8 Search PubMed.
- P. P. Ballesta and E. De Saeger, Diffus. Monit., 1996, 8, 8 Search PubMed.
- N. T. Plant M. D. Wright, European Diffusive Sampling Initiative: Project Report with Status at March 1998, Report IACS/98/01, Health and Safety Laboratory, Sheffield, 1998 (obtainable from the Health and Safety Laboratory, Broad Lane, Sheffield, UK S3 7HQ). Search PubMed.
- R. H. Brown K. J. Walkin, Anal. Proc., 1981, 18, 205. Search PubMed.
- International Standards Organization, Ambient, Indoor and Workplace Air – Determination of Concentrations of Volatile Organic Compounds in Air, Thermal Desorption Method – Part 2: Diffusive Sampling Method, ISO/DIS 16017-2, ISO, Geneva, 1999. Search PubMed.
- G. Bertoni, S. Canepari, M. Rotatori, R. Fratarcangelli and A. Liberti, J. Chromatogr., 1990, 522, 285 CrossRef CAS.
- X. L. Cao and C. N. Hewitt, Environ. Sci. Technol., 1994, 28, 757 CAS.
- D. Helmig, J. Chromatogr. A., 1996, 732, 414 CrossRef CAS.
- W. A. Boyle, Diffus. Monit., 1998, 10, 19 Search PubMed.
- P. P. Ballesta R. A. Field E. De Saeger, Field Intercomparison of VOC Measurements, JRC Report EUR 18085 EN European Commission, Brussels, 1998. Search PubMed.
- R. H. Brown, J. Environ. Monit., 1999, 1, 115 RSC.
- B. Striefler, Diffus. Monit., 1993, 6, 9 Search PubMed.
- A. Hanus-Illnar, Drägerheft, 1997, 365, 32 Search PubMed.
- Der Eisatz von Passivsammlern zur Ermittelung der Benzolbelastung in Strassenschluchten, Niedersächisches Landesamt für Ökologie, Hannover, 1997. Search PubMed.
- Luftschadstoffbelastung in Strassenschluchten, Niedersächisches Landesamt für Ökologie, Hannover, 1994. Search PubMed.
- B. Heits, W. Busch, K.-P. Geisen, W. J. Müller, B. Striefler, D. Bürger and H. Linde, VDI Ber., 1996, 1257, 313 Search PubMed.
- G. W. Wooten J. E. Strobel J. V. Pustinger C. R. McMillin J. D. Mulik, High Performance Passive Sampling Device for Ambient Air and Short-term Personal Sampling Applications, Monsanto Report MRC-DA-1147, Monsanto, Dayton, OH. Search PubMed.
- G. W. Wooten J. E. Strobel J. V. Pustinger C. R. McMillin, Passive Sampling Device for Ambient Air and Personal Sampling, EPA Report 600/4-84-050, US Environmental Protection Agency, Research Park Triangle, NC, USA, 1984. Search PubMed.
- R. W. Coutant, R. G. Lewis and J. D. Mulik, Anal. Chem., 1985, 57, 219 CrossRef.
- R. W. Coutant, R. G. Lewis and J. D. Mulik, Anal. Chem., 1986, 58, 445 CrossRef CAS.
- J. L. Varns J. D. Mulik D. Williams, in Proceedings of 1990 EPA/A and WMA International Symposium on Measurements of Toxic and Related Air Pollutants, Raleigh, NC. Search PubMed.
- R. W. Coutant and D. R. Scott, Environ. Sci. Technol., 1982, 16, 410 CAS.
- M. A. Cohen, P. B. Ryan, Y. Yanagisawa and S. K. Hammond, J. Air Waste Manage. Assoc., 1990, 40, 993 Search PubMed.
- H. C. Schields and C. J. Weschler, J. Air Pollut. Control Assoc., 1987, 37, 1039 Search PubMed.
- M. Bates, N. Gonzalez-Flesca, V. Cocheo and R. Sokhi, Analyst, 1997, 122, 1481 RSC.
- J. O. Levin, R. Lindahl and K. Andersson, Environ. Sci. Technol., 1986, 20, 1273 CAS.
- J. O. Levin, R. Lindahl and K. Andersson, J. Air Pollut. Control Assoc., 1989, 39, 44 Search PubMed.
- D. Grosjean and E. L. Williams, Atmos. Environ., 1992, 26, 2923.
- J. O. Levin, R. Lindahl and K. Andersson, Environ. Technol. Lett., 1988, 9, 1423 Search PubMed.
- K. Ishii I. Aoki, Determination of Atmospheric Formaldehyde Using a Diffusion Sampler, 1988 Annual Report, Tokyo Environmental Science Research Centre, Tokyo, 1988. Search PubMed.
- R. R. Arnts and S. B. Tejada, Environ. Sci. Technol., 1989, 23, 1428 CAS.
- J. O. Levin, R. Lindahl, C. E. M. Heeremans and K. van Oosten, Analyst, 1996, 121, 1273 RSC.
- Th. Hafkenscheid, Report to BCR, in the press. Search PubMed.
- M. Hangartner, VDI Ber., 1990, 838, 515 Search PubMed.
- K. E. Preschler and M. Schöndube, Haustech. Bauphys. Umwelttech., 1983, 4, 198 Search PubMed.
- M. Kirchner R. Kammerlohr B. Kohmanns G. Redis M. Schödl G. Welzt, Erfassung der Immissionsbelastung im Alpenraum mit Passivsammlern, Materialien Umwelt und Entwicklung Bayern, 116, Bayerisches Staatsministerium für Landesentwicklung und Umweltfragen. Search PubMed.
- J. Striedner, Passive Messmethoden für de Analyse der Luftschadstoffe NO2, SO2 und O3 Mittels Diffusionssammlern, Umweltbundesamt, Vienna, 1997. Search PubMed.
- D. Grosjean and M. W. M. Hisham, J. Air Waste Manage. Assoc., 1992, 42, 169 Search PubMed.
- D. Grosjean and E. L. Williams, Atmos. Environ., 1992, 26A, 1407 CAS.
- H. Werner, Das Indigopapier – Sensitives Element zum Aufbau von Passivsammlern zur Messung von Ozonimmissionen, Forstl. Forschungsber. Muenchen, 1992, 122. Search PubMed.
- M. Hangartner, M. Kirchner and H. Werver, Analyst, 1996, 121, 1269 RSC.
- C. Monn and M. Hangartner, J. Air Waste Manage. Assoc., 1990, 40, 357 Search PubMed.
- P. Koutrakis, J. M. Wolfson, A. Bunyaviroch, S. E. Froelich and J. D. Mulik, Anal. Chem., 1993, 65, 209 CrossRef CAS.
Footnotes |
| † © Crown copyright. |
| ‡ This paper is based on R. H. Brown, Diffusive sampling, in Clean Air at Work, ed. R. H. Brown, M. Curtis, K. J. Saunders and S. Vandendriessche, EC Publication No. EUR 14214, EC, Brussels, Luxembourg, 1992, pp. 142–148, and R. H. Brown, The use of diffusive samplers for monitoring of ambient air, Pure Appl. Chem., 1993, 65, 1859. |
| This journal is © The Royal Society of Chemistry 2000 |
