Conjugation and proton exchange equilibria. Heteroconjugation constants for the 4-nitrobenzoic acid-substituted phenolate systems in acetonitrile
Abstract
The
heteroconjugation constants ![[K with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004b_20d1.gif) AHA1−
= ([AHA1−]yAHA1−
/[HA][A1−]yA1−) for 4-nitrobenzoic acid (HA)-substituted phenolate
(A1−) systems in acetonitrile (AN) were determined by an electrometric method neglecting the formation of heteroconjugates
with the solvent molecules. The constants referred to as
AHA1−
= ([AHA1−]yAHA1−
/[HA][A1−]yA1−) for 4-nitrobenzoic acid (HA)-substituted phenolate
(A1−) systems in acetonitrile (AN) were determined by an electrometric method neglecting the formation of heteroconjugates
with the solvent molecules. The constants referred to as ![[K with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004b_20d1.gif)
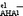 were compared to those
were compared to those ![[K with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004b_20d1.gif)
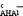 calculated directly from the relationship between homo-, heteroconjugation and proton exchange constants reported
previously. Both lg
calculated directly from the relationship between homo-, heteroconjugation and proton exchange constants reported
previously. Both lg ![[K with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004b_20d1.gif)
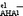 and lg
and lg ![[K with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004b_20d1.gif)
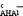 show a fairly linear dependence on ΔpKaAN values referred to as pKHA1AN−pKHAAN. For both lg
show a fairly linear dependence on ΔpKaAN values referred to as pKHA1AN−pKHAAN. For both lg ![[K with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004b_20d1.gif)
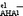 and lg
and lg ![[K with combining right harpoon above (vector)]](https://www.rsc.org/images/entities/i_char_004b_20d1.gif)
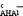 the deviations from this general dependence (caused by
nonequal steric effects during hydrogen bond formation) observed for the same systems are similar in direction
and extent. This finding strongly suggests that the relationship between homo-, heteroconjugation and proton
exchange constants is also a reflection of certain specific properties of the heteroconjugate and may therefore
successfully be used as a ‘standardizing equation
’ for all methods of determining heteroconjugation constants. Some practical comments about the applicability of the electrometric
method in
its current shape are given.
the deviations from this general dependence (caused by
nonequal steric effects during hydrogen bond formation) observed for the same systems are similar in direction
and extent. This finding strongly suggests that the relationship between homo-, heteroconjugation and proton
exchange constants is also a reflection of certain specific properties of the heteroconjugate and may therefore
successfully be used as a ‘standardizing equation
’ for all methods of determining heteroconjugation constants. Some practical comments about the applicability of the electrometric
method in
its current shape are given.

 Please wait while we load your content...
Please wait while we load your content...