Studies of lead(II) complexes of substituted calix[4]diquinones: the remarkable self-assembly of a novel redox-active 3D channel network
Paul D.
Beer
a,
Michael G. B.
Drew
b,
Philip A.
Gale†
a,
Mark I.
Ogden‡
a and
Harold R.
Powell
c
aInorganic Chemistry Laboratory, University of Oxford, South Parks Road, Oxford, UK OX1 3QR
bDepartment of Chemistry, University of Reading, Whiteknights, Reading, UK RG6 6AD. E-mail: m.g.b.drew@reading.ac.uk
cCambridge Crystallographic Data Centre, 12 Union Road, Cambridge, UK CB1 2EZ
Abstract
Several calix[4]diquinone–lead(II) complexes have been prepared. Two momonomeric complexes have been prepared with p-tert-butyl-(26,28-crown-5)calix[4]diquinone L111 and p-tert-butylcalix[4]diquinone bis(ethyl ether) L222, namely [PbL111(ClO4)2] and [PbL222(ClO4)2(H2O] wherein the metal atoms are 10-coordinate being bonded to the four oxygen atoms at the lower rim of the calixdiquinone and three perchlorate oxygen atoms together with three crown oxygen atoms or two carbonyl oxygen atoms and a water molecule respectively. By contrast the lead(II) complex with calix[4]diquinone bis(acid) L333 forms a unique trimeric [Pb9(L333 − 2H)3(ClO4)6(OH)6] unit with crystallographic 3/m symmetry containing three unique Pb(II) atoms. One of these is to be found within the calix[4]diquinone bonded to the four oxygen atoms at the lower rim, two acid oxygen atoms, two perchlorate anions and a water molecule. The other two Pb(II) atoms are bonded to carbonylic oxygen atoms, water molecules and hydroxide ions. These trimeric units are interconnected via upper rim quinone oxygen–Pb(II) interactions to form a three-dimensional network containing channels of ca. 14 Å in diameter.
Introduction
The construction of molecular aggregates containing structures on the nanometre scale is an area of intense current interest.1 In our previous work we have shown that the cation coordination chemistry of substituted calix[4]diquinones is remarkably interesting. Structural variation is observed in these complexes due to the possible involvement of upper rim oxygen atoms in the cation coordination sphere. This feature allows the formation of a variety of structures including dimers and one-dimensional coordination chains.2 Whilst investigating the complexation and redox chemistry of related calix[4]diquinones with cations,3–6 we have discovered a novel example of a continuous three dimensional channel network structure formed by a unique calix[4]diquinone bis-acid L–lead(II) complex which self-assembles via both upper and lower rim quinone oxygen–lead(II) cation interactions.Experimental
L111 and L222 were synthesised via literature methods.2,7–9Crystals of the lead perchlorate complex of L111 were grown by slow evaporation from a dichloromethane–ethanol solvent mixture of L111 in the presence of excess Pb(ClO4)2.xH20.
Crystals of the lead perchlorate complex of L222 were also obtained by slow evaporation of a CH2Cl2 –EtOH solution of the ligand in the presence of excess Pb(ClO4)2.xH2O.
Synthesis of L333: p-tert-butylcalix[4]arene bis-acid10(1.0 g, 1.3 mmol) was stirred in Tl(OCOCF3)3/TFA solution (9 ml, 7.9 mmol) for 2 h in the dark. The TFA was then removed in vacuo and the residue poured onto ice–water (50 ml). The product was then extracted with chloroform (100 ml) and then washed with water (100 ml). A solution of [2.2.1] cryptand (100 mg) in CHCl3 (1 ml) was added to the organic layer and the solution washed with water (10 × 100 ml) and then HCl(aq) (2 × 100 ml, 1.0 M) and then with water (100 ml). The product was isolated by slow evaporation from a mixture of hexane and dichloromethane and precipitated as a yellow powder (0.41g, 46%).
δ
1H NMR (CD2Cl2, 500 MHz): 1.01 (s, 18H, (CH3)3C), 3.09 (d, 2J = 13.2 Hz, 4H, ArCH2Qu : Heq), 4.20 (d, 2J = 13.2 Hz, 4H, ArCH2Qu : Hax), 4.31 (s, 4H, OCH2), 5.31 (CH2Cl2), 6.70 (s, 4H, QuH), 6.76 (s, 4H, ArH). δ 13C NMR + DEPT (CDCl3, 125.7 MHz): 30.23 (ArCH2Qu), 31.71 (CH3C), 34.08 (CH3C), 73.39 (OCH2), [125.61, 129.15, 132.54, 147.30, 148.56, 151.15 (Ar/Qu)], 173.79 (COOH), 186.69 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O), 187.75 (C
O), 187.75 (C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) O). Microanalysis: calculated (C40H40O10): C 70.57 H 5.92; found: C 69.54 H 6.03%. FAB MS (m/z): MH+
@ 681, MNa+
@ 703, MK+ @ 719. Melting point: 225–230
O). Microanalysis: calculated (C40H40O10): C 70.57 H 5.92; found: C 69.54 H 6.03%. FAB MS (m/z): MH+
@ 681, MNa+
@ 703, MK+ @ 719. Melting point: 225–230![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C (dec.).
°C (dec.).
The lead(II) complex of L333 was formed by forming a suspension of L333 in dichloromethane and adding an ethanol solution containing excess Pb(ClO4)2.xH2O to it. The acid would dissolve upon addition but within seconds a yellow precipitate formed which was allowed to settle and the supernatant liquid was transferred to a sample tube. Crystals grew in this solution by slow evaporation of solvent.
Microanalysis: calculated (C40H38O10)3Pb9(ClO4)6(OH)6.16H2O: C 29.49 H 3.13; found: C 28.17 H 3.00%. Microanalysis data for calixarene complexes are notoriously unreliable and therefore these results should be treated with caution.11,12
Crystallography
Crystal data for 1, 2 and 3 are given in Table 1. Data for 1 and 3 were collected with MoKα radiation using the MARresearch Image Plate System. The crystals were positioned at distances from the Image Plate of 75 mm. 95 frames were measured at 2° intervals with a countιng time of 5 min for 1 and 3 and 3 min for 2. Data analyses were carried out with the XDS program.13 Data for 2 were collected with MoKα radiation using the Rigaku R-Axis Image Plate Detector. The crystal was positioned at 80 mm from the Image Plate. 90 frames were measured at 2° intervals with a counting time of 3 min. The three structures were solved using direct methods with the Shelx8614 program for 1 and 3 and the SIR program14 for 2. Empirical absorption corrections were carried out using the DIFABS program.15 Default refinement technique included the refinement of all non-hydrogen atoms anisotropically while hydrogen atoms were included in calculated positions and given thermal parameters equivalent to 1.2 times those of the atoms to which they were bonded. In 1 in addition to the lead complex there was one dichloromethane solvent molecule refined anisotropically and two methanol solvent molecules refined isotropically. In 2 one of the tert-butyl groups was disordered over two sets of tetrahedral sites. These atoms were refined isotropically with occupancy factors of x and 1 − x. x refined to 0.60(2). A terminal methyl group in one of the ethyl groups was disordered over two sites and treated similarly. x refined to 0.54(4). In 3 the calix[4]diquinone had imposed mirror symmetry through two of the phenyl rings. The two unique tert-butyl groups of the calix[4]diquinone were both disordered around this mirror plane and were refined as tetrahedral groups with 50% occupancy. Pb(2) showed high thermal motion perpendicular to the mirror plane and it was investigated whether it was preferable to treat the atom as disordered over two sites at ca. 0.2 Å apart either side of the mirror plane, but as there was no significant reduction in the R value, the metal was kept on the mirror plane. Pb(3) was more obviously disordered over two sites ca. 2 Å apart either side of the mirror plane. It may well be that this position masked a similarly disordered oxygen atom but this was not included in the refinement. There were many additional water and/or solvent molecules in the asymmetric unit, some of which were bonded to the lead atoms and some not. All were refined with reduced occupancies. In the axial positions to Pb(2) (above and below the mirror plane) was an oxygen atom OW(21) which was refined with 50% occupancy and the site was interpreted as being disordered between an oxygen atom and a lone pair. Inside the cavity formed by the three calixdiquinones were several peaks which were refined as water molecules with reduced occupancy (OW(11), OW(22) which were within bonding distance of Pb(1) and Pb(2) respectively) and OW(1) (which was unattached) with 50% occupancy. Six other peaks of electron density were refined as water molecules with occupancies ranging between 25 and 50%. Pb(3) was bonded to two oxygen atoms O(31) and O(32), also given 50% occupancy and interpreted as disordered hydroxide anions although the distinction between hydroxide ions and water molecules could not be definitive. Solvent molecules were refined isotropically and hydrogen atoms were not included. 1 and 3 were refined on F2 using Shelxl16 while 2 was refined on F using Crystals17 to convergence.| 1 | 2 | 3 | |
|---|---|---|---|
| a Click b007061k.txt for full crystallographic data (CCDC no. 1350/36). | |||
| Empirical formula | C46H57Cl4O19Pb | C43H48Cl2O20Pb | C120H122Cl6O70Pb9 |
| M | 1262.9 | 1175.0 | 4761.6 |
| Crystal system, space group | Monoclinic, P21/c | Monoclinic, P21/n | Trigonal, P![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c1 c1 |
| a/Å | 16.91(2) | 18.530(2) | 28.610(9) |
| b/Å | 14.077(15) | 24.901(3) | 28.610(9) |
| c/Å | 20.99(2) | 11.385(1) | 18.390(8) |
| β/° | 92.16(1) | 98.573(5) | — |
| V/Å3 | 4993 | 5194 | 13036 |
| Z, calculated density/Mg m−3 | 4, 1.680 | 4, 1.50 | 2, 1.213 |
| Absorption coefficient/mm−1 | 3.669 | 3.42 | 5.91 |
| R collected/Runique [Rint] | 15156/8107 [0.0633] | 4843 | 22063/6050 [0.0930] |
| Data/restraints/parameters | 8107/0/632 | 4843/0/571 | 6050/18/358 |
| Final R indices [I > 2σ(I)] R1, wR2 | 0.1047, 0.2365 | 0.0823 | 0.1179, 0.2972 |
| R indices (all data) | 0.1373, 0.2531 | 0.0823 | 0.1759, 0.3435 |
| Largest diff. peak and hole/e Å−3 | 1.965, −1.642 | 1.789, −1.840 | 4.595, −2.815 |
Results and discussion
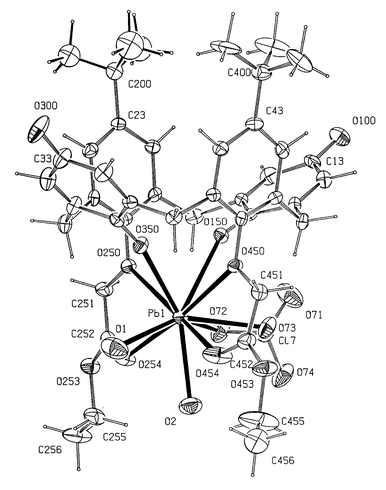
The lead ion in 1 (Fig. 1) is ten-coordinate being bound to the five oxygen atoms in the crown, two lower rim quinone oxygens and three perchlorate oxygens from one bidentate and one monodentate anion. The metal atom and five oxygen atoms in the crown are almost coplanar with deviations from the PbO5 plane of −0.09 for O(250), 0.05 O(253), 0.03 O(256) , −0.12 O(259), 0.09 O(450) and 0.05 Å for Pb(1) (Table 2). Bond lengths to the oxygen atoms in the ring range from 2.580(12) to 2.811(9) Å with the longest bonds to the oxygen atoms at the lower rim of the calix[4]arene. The two quinone oxygen atoms O(150) and O(350) at 2.706(12), 2.592(11) Å are situated either side of the ring plane as indeed are the perchlorate anions. The bond to the monodentate perchlorate anion, Pb–O(72) 2.54(3) Å, is significantly shorter than those involving the bidentate perchlorate oxygen atoms, Pb–O(63) 2.87(2) and Pb–O(64) 2.92(2) Å. The calix[4]diquinone has the distorted cone conformations. The angles subtended by the six-membered rings with the plane of the four methylene groups are 30.6, 83.0, 28.4 and 79.9° for rings 1–4, respectively. | ||
| Fig. 1 The structure of 1 together with the numbering scheme. Ellipsoids shown at 30% occupancy. Click image or Fig1.htm to access 3D representation. Portions of the structure show disorder. | ||
| Bond | Length/Å |
|---|---|
| a Symmetry transformations used to generate equivalent atoms: #1 − x + y + 1,−x + 1,z #2 x,y,−z + 1/2. | |
| 1 | |
| Pb(1)–O(256) | 2.580(12) |
| Pb(1)–O(350) | 2.592(11) |
| Pb(1)–O(72) | 2.60(3) |
| Pb(1)–O(259) | 2.630(10) |
| Pb(1)–O(253) | 2.698(10) |
| Pb(1)–O(150) | 2.706(12) |
| Pb(1)–O(250) | 2.721(9) |
| Pb(1)–O(450) | 2.811(9) |
| Pb(1)–O(63) | 2.876(16) |
| Pb(1)–O(64) | 2.92(2) |
| 2 | |
| Pb(1)–O(1) | 2.484(16) |
| Pb(1)–O(2) | 2.578(13) |
| Pb(1)–O(454) | 2.541(11) |
| Pb(1)–O(254) | 2.635(11) |
| Pb(1)–O(350) | 2.726(11) |
| Pb(1)–O(73) | 2.781(12) |
| Pb(1)–O(150) | 2.783(9) |
| Pb(1)–O(250) | 2.876(8) |
| Pb(1)–O(450) | 2.946(9) |
| Pb(1)–O(72) | 2.986(13) |
| 3 | |
| Pb(1)–O(254) | 2.51(3) |
| Pb(1)–O(454) | 2.60(2) |
| Pb(1)–OW(11) | 2.64(5) |
| Pb(1)–O(350) | 2.745(13) |
| Pb(1)–O(61) | 2.69(4) |
| Pb(1)–O(62) | 2.88(4) |
| Pb(1)–O(450) | 2.845(17) |
| Pb(1)–O(250) | 2.960(14) |
| Pb(2)–OW(22) | 2.51(5) |
| Pb(2)–O(253) | 2.484(17) |
| Pb(2)–O(453)#1 | 2.41(2) |
| Pb(2)–OW(21) | 2.61(6) |
| Pb(2)–O(254) | 2.72(2) |
| Pb(2)–O(454)#1 | 2.73(2) |
| Pb(3)–O(32) | 2.49(7) |
| Pb(3)–O(31) | 2.59(6) |
| Pb(3)–O(253) | 2.58(2) |
| Pb(3)–O(453)#1 | 2.74(2) |
| Pb(3)–O(300)#2 | 2.954(16) |
A similar conformation is observed in 2 (Fig. 2) where the angles are 33.0, 83.2, 39.9 and 81.9°. In our previous work2 on cation structures of calix[4]quinones, we found that this particular type of cone conformation with the aromatic rings more perpendicular and the quinone rings more parallel to the plane of the four methylene atoms was found in all six structures with angles ranging from 78.8–94.5 and 27.1–40.4° respectively. The values reported here fall within this common range. This conformation is due primarily to the fact that C–O–M angles subtended at the oxygen atoms in the lower rim are very different for ether oxygens and for quinone oxygen atoms. Thus in 1, the C–O–Pb angles are 126.1(7), 127.9(8) and 164.7(11), 155.4(11)°, respectively.
 | ||
| Fig. 2 The structure of 2 together with the numbering scheme. Ellipsoids shown at 30% occupancy. Click image or Fig2.htm to access 3D representation. | ||
In 2 the lead cation is bonded to ten oxygen atoms, six from the calix[4]diquinone, together with two water molecules Pb(1)–O(1) 2.48(2), Pb(1)–O(2) 2.58(1) Å and two from a chelating perchlorate counter anion Pb(1)–O(72) 2.99(2) and Pb(1)–O(73) 2.77(2) Å. As in 1 the cations are discrete and not linked through the upper rim quinone oxygen atoms to form dimers or indeed chains as has been found previously in a range of structures with this ligand and related derivatives. The distances from the lead atom to the lower rim oxygen atoms are 2.783(11), 2.726(12) Å for the quinone oxygen atoms O(150), O(350) and 2.877(14), 2.946(14) Å for the ether oxygens O(250), O(450). In comparison, the bonds to the carbonyl oxygens O(254), O(454) are very much shorter at 2.635(11), 2.541(11) Å.
By contrast with these monomeric lead complexes 1 and 2, the structure of 3 consists of trimeric [Pb9(L − 2H)3(ClO4)6(OH)6] units with crystallographically imposed 3/m symmetry (Fig. 3) together with assorted disordered solvent. There are three independent lead atoms in the structure. Pb(1) is enclosed within the calix[4]arene in a similar manner to that found for the metal atoms in 1 and 2 and is situated on the mirror plane which also intersects the two phenyl rings of the calix[4]diquinone. The metal atom is ten or eleven coordinate, being bonded to the four oxygen atoms at the bottom rim O(350)*2 at 2.745(13) Å, O(250) at 2.960(14) Å and O(450) at 2.845(17) Å and to two carboxylate oxygen atoms O(254) 2.51(3) Å and O(454) 2.60(2) Å. In addition Pb(1) is bonded in a bidentate fashion to two perchlorate anions O(61), 2.69(4), O(12) 2.88(5) Å above and below the equatorial mirror plane. There is also an interaction of 2.64(5) Å with OW(11), a disordered water molecule with 50% occupancy.
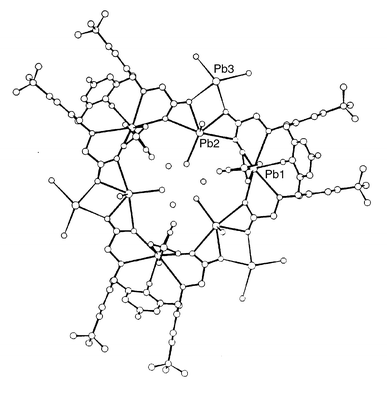 | ||
| Fig. 3 The trimeric structure of 3 together with the numbering scheme. Click image or Fig3.htm to access 3D representation. The structure shows disorder. | ||
The calix[4]quinone adopts the usual distorted cone conformation with aromatic rings pseudo-perpendicular and quinone rings pseudo-parallel to the plane of the four methylene atoms (angles of intersection 88.3, 80.2; 2 × 29.0°). Both carboxylate groups are perpendicular to the phenyl ring to which they are attached and are therefore fixed on the mirror plane.
Three of these PbL333 (ClO4)2 units are bridged around a crystallographic 3-fold axis via Pb(2) which is bonded to two bidentate carboxylic acid groups in adjacent PbL333 (ClO4)2 units with bond lengths of 2.484(17) to O(253), 2.72(2) to O(254), 2.73(2) to O(454) and 2.41(2) Å to O(453). Thus the bond lengths to the carboxylic acid oxygen atoms shared with Pb(1), e.g. O(254) and O(454), are much longer than those to O(253) and O(453) although these latter are shared with Pb(3) (see later). The equatorial plane is completed by OW(22) at 2.51(5) Å, the bond to which is directed towards the centre of the cavity at the three-fold axis but like OW(11) similarly attached to Pb(1), this atom has only 50% occupancy. This PbO5 unit is crystallographically planar although the thermal parameters of Pb(2) indicate that the position could be disordered either side of the mirror plane. The axial sites are occupied by one oxygen atom OW(22) with 50% occupancy which was interpreted as indicating disorder between one oxygen at 2.61(6) Å and one lone pair. In structures of this type, the waterax–Pb–Oeq angles are <90° and the lonepairax–Pb–Oeq angles are >90° which explains the lead disorder.11,12
It seems likely that this disorder must arise because the small size of the cavity is insufficient for all the water molecules to co-exist and also because of the presence of the stereochemically active lone pair.
Pb(3) is also disordered over two sites ca. 2 Å apart, occurring either side of the mirror plane. The impetus for the disorder presumably arises from the disorder in the position of Pb(2). Pb(3) is bonded to two acid oxygen atoms O(253) 2.58(2) Å and O(453) 2.743(19) Å and to two further oxygen atoms O(31) and O(32) at 2.59(6) and 2.49(7) Å. The latter oxygen atoms are given 50% occupancy in common with Pb(3). They are monodentate and have high thermal parameters. There are other atoms in close contact with O(31) and O(32) (apart from Pb(3)) and this may be the cause of the high thermal motion of these two atoms They have been refined as OH groups in order to provide charge balance for the trimer. The Pb(3) ion is also bonded to an additional oxygen atom O(300) {2.954(16) Å} at the top of the cone of a calix[4]diquinone unit in an adjacent trimer.
As shown in Fig. 3, the [Pb9(L333 − 2H)3(ClO4)6(OH)6] trimer contains a small channel around the 3-fold axis at 2/3, 1/3, z and filled with disordered solvent molecules. In addition there is a much larger channel around 0,0,z (Fig. 4). Three trimers with the same z coordinate pack around this channel and then three more at z + 0.5 connected to the three trimers at z with the aforementioned Pb(3)⋯O(300) interactions to form a large channel bordered by six trimers. This channel is relatively free of significant electron density although the presence of highly mobile and thus disordered solvent molecules cannot be ruled out.
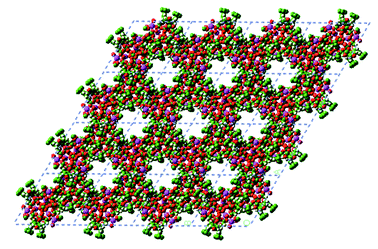 | ||
| Fig. 4 Crystal packing of 3 in the z projection showing the presence of small channels within the trimer and large channels between trimers. Click image or Fig4.htm to access 3D representation. | ||
Work is currently underway to incorporate neutral species into the channels present in the crystal and we are also investigating the formation of other continuous supramolecular arrays formed from metal cation–calixdiquinone complexes.
We thank the EPSRC for a studentship to PAG and for the use of the Mass Spectrometry Service at the University of Wales, Swansea, and the EPSRC together with the University of Reading for Provision of the Image Plate System.
References
- (a) G. M. Whitesides, J. P. Mathias and C. T. Seto, Science, 1991, 254, 1312 CrossRef CAS PubMed and references cited therein.; (b) J. Yang, J.-L. Marendaz, S. J. Geib and A. D. Hamilton, Tetrahedron Lett., 1994, 35, 3665 CrossRef CAS and references cited therein.; (c) L. R. Barbar, G. W. Orr and J. L. Atwood, Nature, 1998, 393, 671 CrossRef; (d) L. R. MacGillivray and J. L. Atwood, Nature, 1997, 389, 469 CrossRef CAS; (e) L. R. MacGillivray and J. L. Atwood, Science, 1999, 285, 1049 CrossRef; (f) T. Kusukawa and M. Fujita, Angew. Chem., Int. Ed., 1998, 37, 3142 CrossRef CAS; (g) O. D. Fox, M. G. B. Drew and P. D. Beer, Angew. Chem., Int. Ed., 2000, 39, 135 CrossRef; (h) O. D. Fox, M. G. B. Drew, E. J. S. Wilkinson and P. D. Beer, Chem. Commun., 2000, 391 RSC.
- P. B. Beer, P. A. Gale, Z. Chen, M. G. B. Drew, J. A. Heath, M. I. Ogden and H. R. Powell, Inorg. Chem., 1997, 36, 5880 CrossRef CAS PubMed.
- P. D. Beer, Z. Chen and P. A. Gale, Tetrahedron, 1994, 50, 931 CrossRef CAS.
- P. D. Beer, Z. Chen, M. G. B. Drew and P. A. Gale, J. Chem. Soc., Chem. Commun., 1994, 2207 RSC.
- P. D. Beer, Z. Chen, M. G. B. Drew, P. A. Gale, J. A. Heath, R. J. Knubley and M. I. Ogden, J. Inclusion Phenom. Mol. Recognit. Chem., 1994, 19, 343 CrossRef CAS.
- Z. Chen, P. A. Gale, J. A. Heath and P. D. Beer, J. Chem. Soc., Faraday Trans., 1994, 90, 2931 RSC.
- A. McKillop, B. P. Swann and E. C. Taylor, Tetrahedron, 1970, 26, 4031 CrossRef CAS.
- P. A. Reddy, R. P. Kashyap, W. H. Watson and C. D. Gutsche, Isr. J. Chem., 1992, 32, 89 CrossRef CAS.
- P. A. Reddy and C. D. Gutsche, J. Org. Chem., 1993, 58, 3245 CrossRef CAS.
- E. M. Collins, M. A. McKervey, E. Madigan, M. B. Moran, M.Owens, G. Ferguson and S. J. Harris, J. Chem. Soc., Perkin Trans., 1991, 1, 3137 RSC.
- (a) C. D. Gutsche and K. A. See, J. Org. Chem., 1992, 57, 4527 CrossRef CAS; (b) V. Böhmer, K. Jung, M. Schön and A. Wolff, J. Org. Chem., 1992, 57, 790 CrossRef.
- H. von. Arnim, K. Dehnicke, K. Maczek and D. Fenske, Z. Anorg. Allg. Chem., 1993, 619, 1704 CrossRef.
- W. Kabsch, J. Appl. Crystallogr., 1988, 21, 916 CrossRef CAS.
- (a) G. M. Sheldrick, Acta Crystallogr., Sect. A, 1990, A46, 467 CrossRef CAS; (b) A. Altomare, G. Cascarano, C. Giacovazzo and A. Guagliardi, J. Appl. Crystallogr., 1993, 26, 343 CrossRef.
- N. Walker and D. Stuart, Acta Crystallogr., Sect. A, 1983, A39, 158 CrossRef CAS.
- Shelxl, Program for Crystal Structure Refinement, G. M. Sheldrick, University of Göttingen, 1993. Search PubMed.
- Crystals, issue 10, D. J. Watkin, C. K. Prout, J. R. Carruthers and P. W. Betteridge, Chemical Crystallography Laboratory, University of Oxford, Oxford, UK, 1996. Search PubMed.
Footnotes |
| † Present address: Department of Chemistry, University of Southampton, Southampton, UK SO17 1BJ. |
| ‡ Present address: School of Applied Chemistry, Curtin University of Technology, Perth, Western Australia. |
| This journal is © The Royal Society of Chemistry 2000 |
