Crystal engineering of coordination polymers and architectures using the [Cu(2,2′-bipy)]2+ molecular corner as building block (bipy = 2,2′-bipyridyl)
Lucia Carluccia, Gianfranco Ciani*b, Alberto Gramacciolib, Davide M. Proserpiob and Silvia Rizzatob
aDipartimento di Biologia Strutturale
e Funzionale, Università dell’Insubria, Via J. H. Dunant 3, 21100, Varese, Italy
bDipartimento di Chimica Strutturale
e Stereochimica Inorganica and Centro CNR, Via G. Venezian 21, 20133, Milano, Italy. E-mail: davide@csmtbo.mi.cnr.it
Abstract
The [Cu(2,2′-bipy)]2+ corner unit (1) (2,2′-bipy = 2,2′-bipyridyl), made available by the metathesis of the chlorides in [Cu(2,2′-bipy)Cl2] with poorly coordinating anions [triflate (1a), BF4− (1b) and NO3− (1c)], has been reacted with a variety of bifunctional ligands, such as 4,4′-bipyridyl (4,4′-bipy), trans-4,4′-azobis(pyridine) (azpy), 1,2-bis(4-pyridyl)ethane (bpetha), trans-1,2-bis(4-pyridyl)ethene (bpethe), 1,3-bis(4-pyridyl)propane (bpp), pyrazine (pyz), i-nicotinate (i-nic) and others. Interesting motifs have been obtained and characterized, including one-dimensional polymeric chains of different kinds, molecular rings and other architectures, depending on the ligand and the counterion. Zig-zag chains exhibiting a variated period have been observed within the species [(1)(4,4′-bipy)(CF3SO3)](CF3SO3) (2a), [(1)(azpy)(H2O)(CF3SO3)](CF3SO3) (3a), [(1)(bpethe)(BF4)](BF4) (2b), [(1)(azpy)(H2O)](NO3)2·H2O (2c) and [(1)(i-nic)(H2O)](NO3)·2H2O (3c). Though the topology of these species is not exceptional, one of them (2c) exhibits a peculiar supramolecular organization of the chains, resulting in warp-and-woof two-dimensional sheets. With the non-rigid ligands bpp and bpetha other motifs can be obtained: the former ligand gives a festoon polymer, [(1)(bpp)(EtOH)](BF4)2 (3b), while molecular rings are generated with the latter one in the species [(1)(bpetha)(CF3SO3)2]2 (4a). Pyrazine with 1a gives dinuclear species that are joined by triflate anions into ladder-like polymers in [{(1)(H2O)(CF3SO3)}2(pyz)](CF3SO3)2 (5a), exhibiting in the crystal structure channels running parallel to the ladders. With the asymmetric ligand 2,4′-bipyridyl (2,4′-bipy) the monomeric species [(1)(2,4′-bipy)(H2O)(BF4)2] (4b) is obtained, that forms 1D chains via hydrogen bonds. A different starting species, obtained from [Cu(2,2′-bipy)Cl2] by partial metathesis of the chlorides was also employed: reaction with heptanedinitrile (hdn) gives the derivative [{(1)Cl}2(hdn)](BF4)2 (5b), consisting of chains of dinuclear [(2,2′-bipy)Cu(μ-Cl)2Cu(2,2′-bipy)] moieties joined by the bidentate flexible ligand.
Introduction
The use of predetermined building blocks is of basic relevance in two areas of great current interest dealing, respectively, with the deliberate construction of coordination networks1 and with the design of metal-based supramolecular architectures.2 Metal containing subunits with programmed coordination angles can be employed for the self-assembly of frameworks and of macromolecular polygons and polyhedral cages, of potential utility for molecular recognition. Many interesting cyclic nanostructures have been obtained in the last years by Fujita,3 Stang4 and many others,3–5 using rational synthetic methods. In these architectures the most frequently employed metal ‘corners’ were cis-PdII and cis-PtII square planar complexes, particularly for the building of supramolecular squares. However, though the self-assembly of discrete macrocyclic architectures is favoured under thermodynamic equilibration and can be highly convergent, the competitive generation of open-chain oligomers or polymers is often observed.6 We report here on the use of a different corner unit, namely [Cu(2,2′-bipy)]2+ (1), that exhibits two cis-equatorial and two axial free coordination sites. Unit 1, with triflate (1a), tetrafluoroborate (1b) or nitrate (1c) as counterions, has been reacted with different bifunctional rigid or flexible, neutral or anionic spacer ligands. Our hypothesis was to obtain derivatives with the entering ligands coordinated to 1 in the two equatorial positions (see Scheme 1) and with one or both the axial positions involved in weaker interactions with the counterions or solvent molecules. Possible target motifs are illustrated in Scheme 1, including macromolecules and polymers, some of which (I–IV) have been ideally associated with the (not strictly necessary) use of rigid spacers, while the remaining ones (V–VIII) need surely more flexible ligands. In this preliminary study we have operated in conditions that have favoured the kinetic products, thus obtaining mainly, but not exclusively, polymeric species. | ||
| Scheme 1 | ||
Experimental section
Synthesis of the parent species
The starting complex [Cu(2,2′-bipy)Cl2] was prepared according to the method described in the literature,7 by addition of an ethanolic solution of the 2,2′-bipy ligand to an equimolar amount of CuCl2 dissolved in ethanol. The reaction product, precipitated from the reaction mixture, was recovered by filtration, washed with ethanol and dried at 110![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C for a night. (Yield: ca.
76%.) Elem. anal. calc. for C10H8Cl2Cu:
C = 41.33%; H = 2.75%;
N = 9.64%. Found: C = 41.1%;
H = 2.62%; N = 9.41%.
°C for a night. (Yield: ca.
76%.) Elem. anal. calc. for C10H8Cl2Cu:
C = 41.33%; H = 2.75%;
N = 9.64%. Found: C = 41.1%;
H = 2.62%; N = 9.41%.Ethanolic solutions of 1a and 1b and aqueous solutions of 1c were prepared by treatment of [Cu(2,2′-bipy)Cl2] with 2.1 eq. of, respectively, AgSO3CF3, AgBF4 and AgNO3 in the appropriate solvent. The removal of AgCl by filtration or centrifugation gave solutions about 0.01 M of 1a, 1b and 1c, which were directly used in the crystallization experiments described below.
Isolation of the derivatives
All the derivatives obtained and characterized are listed in Table 1. Crystallizations of 1a with the different spacer ligands to give 2a–5a were performed by carefully layering the ethanolic solution of 1a over a dichloromethane solution of the ligand, at room temperature in the molar ratio of 1∶1. Crystals were grown by slow diffusion or by slow evaporation of the solvents. In a similar way we have reacted ethanolic solution of 1b with dichloromethane solutions of the ligands, giving products 2b–4b, by using metal-to-ligand molar ratios of 1∶1 (2b), 1∶3 (3b) and 1∶2 (4b).| Compound | Structure type |
|---|---|
| a Compounds 2–5a, 2–5b and 2–3c contain as counterions triflate, tetrafluoroborate and nitrate, respectively.b (1) = Cu(2,2′-bipy).c The ligands are abbreviated as follows: 2,2′-bipy = 2,2′-bipyridyl, 4,4′-bipy = 4,4′-bipyridyl; azpy = trans-4,4′-azobis(pyridine), bpetha = 1,2-bis(4-pyridyl)ethane, bpethe = trans-1,2-bis(4-pyridyl)ethene, bpp = 1,3-bis(4-pyridyl)propane, pyz = pyrazine, 2,4′-bipy = 2,4′-bipyridyl, hdn = heptanedinitrile, i-nic = i-nicotinate. | |
| [(1)(4,4′-bipy)(CF3SO3)](CF3SO3) (2a) | 1D zig-zag chains |
| [(1)(azpy)(H2O)(CF3SO3)](CF3SO3) (3a) | 1D zig-zag chains joined by H-bonds |
| [(1)(bpetha)(CF3SO3)2]2(4a) | Molecular rings |
| [{(1)(H2O)(CF3SO3)}2(pyz)](CF3SO3)2 (5a) | Dinuclear units bridged into ladders by anions |
| [(1)(bpethe)(BF4)](BF4) (2b) | 1D zig-zag chains bridged by anions |
| [(1)(bpp)(EtOH)](BF4)2 (3b) | 1D festoon polymers |
| [(1)(2,4′-bipy)(H2O)(BF4)2] (4b) | Monomers giving 1D chains via H-bonds |
| [{(1)Cl}2(hdn)](BF4)2 (5b) | 1D chains of dinuclear (μ-Cl)2-bridged units |
| [(1)(azpy)(H2O)](NO3)2.H2O (2c) | Zig-zag chains giving warp-and-woof sheets |
| [(1)(i-nic)(H2O)](NO3).2H2O (3c) | 1D zig-zag chains |
The crystallization of compound 2c was accomplished by layering on a water solution of 1c an ethanolic solution of azpy in a molar ratio of 1∶1. Compound 3c was obtained by layering on a water solution of 1c an ethanolic solution of 4-pyridyl-i-nicotinate in a molar ratio of 1∶1. Hydrolysis of the ligand leads to the formation of the i-nicotinate anions.
Partial methathesis of the chlorides from [Cu(2,2′-bipy)Cl2] with 1 eq. of AgBF4 gave, after removal by filtration of the AgCl precipitate, an ethanolic solution of [Cu(2,2′-bipy)Cl](BF4) that was used as such in the crystallization with a variety of ligands. Only one crystalline product was isolated, (5b), obtained using a dichloromethane solution of heptanedinitrile (hdn) in a three-fold molar excess.
Crystallography
The crystal data for all the compounds are listed in Table 2. The data collections were performed at 293 K (Mo-Kα λ = 0.71073 Å) on a Siemens SMART CCD area-detector diffractometer for 3a, 5a, 2b and 5b, and an Enraf-Nonius CAD4 for 2a, 4a, 3b, 4b, 2c and 3c, by the ω-scan method, within the limits 3 < θ < 24° (5a, 2b, 3b, 4b, 2c ), 3 < θ < 25° (2a, 4a, 3c), 2 < θ < 26° (3a), 2 < θ < 30° (5b). An empirical absorption correction was applied (SADABS)8 for the structures collected on the SMART CCD, while the ψ-scan method was used for the others. The structures were solved by direct methods (SIR97)9 and refined by full-matrix least-squares (SHELX97).10 Anisotropic thermal parameters were assigned to all the non-hydrogen atoms but not to the disordered ones in some of the structures, that were refined isotropically. Disordered anions were found in compounds 2a, 2b, 3b and 2c and suitable disorder models were refined in all cases, as well as for the complete azpy ligand in 2c where a double model (with half weight atoms) was used. The low diffraction power of 5a and 4b did not allow anisotropic refinement for all atoms and isotropic thermal parameters were used to keep significant observations/parameters ratios. Compounds 5a and 2b contain channels and voids full of highly disordered solvent molecules. The contributions of the disordered solvent to the diffraction pattern, that could not be rigorously included in the crystallographic calculations, were subtracted by the SQUEEZE procedure implemented in the PLATON software.11 All the diagrams were obtained using SCHAKAL99 program.12| Properties | 2a | 3a | 4a | 5a | 2b | 3b | 4b | 5b | 2c | 3c |
|---|---|---|---|---|---|---|---|---|---|---|
| a Weighting: w/1/[σ2(Fo2) + (aP)2 + bP] where P/(Fo2 + 2Fc2)/3; criterion for observed reflections: I > 2σ(I). Click b006306l.txt for full crystallographic data (CCDC no. 1350/34).b R1/Σ||Fo| − |Fc||/Σ|Fo|.c wR2/[Σ w (Fo2 − Fc2)2/Σ wFo4]1/2. | ||||||||||
| Chemical formula | C22H16CuF6N4O6S2 | C22H18CuF6N6O7S2 | C48H40Cu2F12N8O12S4 | C28H24Cu2F12N6O14S4 | C22H18B2CuF8N4 | C25H28B2CuF8N4O | C20H18B2CuF8N4O | C27H26B2Cl2Cu2F8N6 | C20H20CuN8O8 | C16H18CuN4O8 |
| M | 674.05 | 720.08 | 1404.20 | 1151.85 | 575.56 | 637.67 | 567.54 | 806.14 | 563.98 | 457.88 |
| Crystal system | Monoclinic | Triclinic | Triclinic | Orthorhombic | Orthorhombic | Monoclinic | Monoclinic | Monoclinic | Orthorhombic | Monoclinic |
| Space group | P21/m (no. 11) | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2)
(no. 2) | P![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) (no. 2)
(no. 2) | Pccn (no. 56) | Pbcm (no. 57) | P21/m (no. 11) | P21/a (no. 14) | C2/c (no. 15) | Pcan (no. 60) | P21/a (no. 14) |
| a/Å | 9.695(3) | 9.104(1) | 10.023(3) | 24.047(2) | 14.713(1) | 8.823(3) | 15.935(6) | 19.177(2) | 8.436(2) | 12.114(5) |
| b/Å | 13.422(5) | 11.755(1) | 10.048(2) | 13.909(1) | 15.897(1) | 11.962(4) | 7.430(5) | 8.298(1) | 17.100(3) | 11.555(5) |
| c/Å | 10.228(4) | 13.595(1) | 14.509(4) | 14.478(1) | 13.890(1) | 14.220(6) | 20.424(6) | 21.249(3) | 32.766(8) | 14.514(5) |
| α/° | 88.72(1) | 81.76(2) | — | — | — | — | — | — | — | — |
| β/° | 92.20(4) | 87.66(1) | 80.93(2) | — | 95.73(3) | 108.60(3) | 109.80(1) | — | 109.80(4) | — |
| γ/° | 87.33(1) | 81.26(2) | — | — | — | — | — | — | — | — |
| V/Å3 | 1330.0(8) | 1451.8(2) | 1415.5(6) | 4842.5(6) | 3248.8(4) | 1493.3(1) | 2291.8(19) | 3181.5(7) | 4726.7(18) | 1911.5(13) |
| Z | 2 | 2 | 1 | 4 | 4 | 2 | 4 | 4 | 8 | 4 |
| ρcalc/g cm−3 | 1.683 | 1.647 | 1.475 | 1.580 | 1.177 | 1.418 | 1.645 | 1.683 | 1.585 | 1.591 |
| μ/mm−1 | 1.066 | 0.987 | 0.887 | 1.158 | 0.732 | 0.806 | 1.040 | 1.582 | 0.988 | 1.196 |
| R1,b wR2c | 0.0803, 0.2194 | 0.0502, 0.1221 | 0.0347, 0.0838 | 0.0977, 0.2288 | 0.0949, 0.2273 | 0.0858, 0.2336 | 0.0898, 0.2239 | 0.0324, 0.0788 | 0.0902, 0.2442 | 0.0463, 0.1005 |
Results and discussion
The building block [Cu(2,2′-bipy)]2+ (1) has been obtained by reacting [Cu(2,2′-bipy)Cl2] with different silver(I) salts in order to replace the chlorides with the poorly coordinating anions CF3SO3− (1a), BF4− (1b) or NO3− (1c). The parent compound is a one-dimensional polymer comprised of Cu(2,2′-bipy)Cl2 square planar units joined in the apical positions to adjacent units via weak Cu–Cl interactions.13 Elimination of the chlorides creates a ‘90° metal corner’ that can form two stronger bonds in the equatorial directions trans to the N atoms of the 2,2′-bipy ligand and one or two weaker interactions (with the anions or solvent molecules) in the axial directions, due to Jahn–Teller distortions. In other words, 1 could be considered as a pseudo-square planar building block, playing a structural role similar to that of the cis-platinum or cis-palladium square planar species widely employed for the construction of metal-based supramolecular architectures. The structural features of the re-crystallized starting materials 1a and 1b are in perfect agreement with the above description, both displaying a square planar [Cu(2,2′-bipy)(H2O)2]2+ moiety that shows only weak interactions with the two anions in axial positions.14However, other coordination geometries around the copper atom cannot be ruled out and 1 is not so rigid as one could desire: an analysis on the Cambridge Database of all the complexes containing the [Cu(2,2′-bipy)]2+ moiety (33 entries) shows that the range of possible geometries for Cu(II) is rather wide, including, beside octahedral and tetragonally elongated octahedral, many examples of square pyramidal and trigonal bipyramidal species, as well as less neatly defined geometries.
We have reacted at room temperature, using the slow diffusion method, freshly prepared solutions of 1a, 1b and 1c with many bifunctional ligands, mainly containing pyridyl groups as donors (see Scheme 2).
 | ||
| Scheme 2 | ||
The solid products obtained under these conditions, examined under the microscope, showed, in all cases, the presence of a single dominant crystalline species. All the crystalline products isolated (see Table 1) have been characterized by single crystal X-ray analysis. All but two (5a and 5b) are 1∶1 adducts.
The majority of these products, showing an elongated crystal morphology, consists of one-dimensional polymers and only in one case (4a) we have observed the formation of a macrocyclic ring. The competition between cyclic species and open-chain oligomers or polymers has been observed in many related systems. As outlined by Fujita, in studying the self-assembly of [(en)Pd(NO3)2] with pyridine-based bridging ligands, systems under kinetic control disfavour cyclization, while thermodynamic conditions facilitate the formation of macrocycles, due to significant entropy effects that favour small cyclic structures over polymeric structures.6d Cyclization was, therefore, observed to occur more readily in the presence of more labile metal–ligand bonds, at low reagent concentrations and at higher temperatures.6 The reaction conditions that we have employed have quite likely favoured the kinetic products. In the case of 4a the formation of a cycle was probably facilitated by the flexibility of the bpetha ligand.
Compound 3c was rather unexpected. Indeed we have reacted a water solution of 1c with the ad hoc prepared spacer 4-pyridyl-i-nicotinate in ethanol, but the hydrolysis of the ligand has produced i-nicotinate anions.
Compound 5b belongs to a different family in that its parent species is not 1b but a complex of composition [Cu(2,2′-bipy)Cl](BF4), that has not been isolated and structurally characterized. Solutions of this complex were directly employed for reactions with a variety of bidentate ligands, but the only crystalline product that we have, at present, obtained is the derivative with heptanedinitrile (hdn).
The structural features of the compounds reported in Table 1 are described below.
Zig-zag polymeric chains
This is the dominant structural motif, found in five compounds containing different spacer ligands and/or anions, i.e.2a, 3a, 2b, 2c and 3c. Each polymer displays peculiar intrachain and interchain features that will be compared. In particular the intrachain parameters to be examined will be the metal coordination, the ligand conformations, the dihedral angles between the [Cu(2,2′-bipy] plane and the Cu(spacer)2 one, the Cu⋯Cu contacts along the chains and the period of the polymers. The supramolecular organization of the chains and the interchain interactions (π–π stacking, hydrogen bonds) will also be considered. Selected bond parameters for these compounds are given in Table 3. The single chains in the five compounds are illustrated in Fig. 1. A ‘stretching factor’ has been reported for each species, evaluated as the percent ratio of the ‘ideal’ to the real period, where the ‘ideal period’ has been assumed to be equal to 2L sin 45° (L = Cu⋯Cu contact).| Compound | Bond lengths/Å | Bond angles/° | ||||
|---|---|---|---|---|---|---|
| a Donor atoms: N1a and N2a = 2,2′-bipy; N1b and N2b = spacer ligands; O(w) = coordinated water; O(an) = coordinated anion; O(solv) = coordinated solvent.b Second model for the disordered ligand. | ||||||
| 2a | Cu–N1a | 1.999(7) | N1a'–Cu–N1a | 80.9(5) | N1a–Cu–N1b' | 169.5(3) |
| Cu–N1b | 2.009(7) | N1b'–Cu–N1b | 87.3(4) | N1a–Cu–O(an) | 96.3(3) | |
| Cu–O(an) | 2.274(7) | N1a–Cu–N1b | 95.0(3) | N1b–Cu–O(an) | 93.8(3) | |
| 3a | Cu–N1a | 2.001(4) | N1a–Cu–N2a | 81.0(2) | N1a–Cu–O(w) | 93.6(1) |
| Cu–N2a | 2.006(3) | N1b–Cu–N2b | 86.4(2) | N2a–Cu–O(w) | 86.2(1) | |
| Cu–N1b | 2.029(4) | N1a–Cu–N1b | 172.0(2) | N1b–Cu–O(w) | 93.8(1) | |
| Cu–N2b | 2.035(4) | N1a–Cu–N2b | 96.0(2) | N2b–Cu–O(w) | 94.5(2) | |
| Cu–O(w) | 2.360(3) | N2a–Cu–N1b | 96.5(2) | O(w)–Cu–O(an) | 168.1(1) | |
| Cu–O(an) | 2.620(4) | N2a–Cu–N2b | 176.9(2) | N–Cu–O(an) | 81.9–97.3(2) | |
| 4a | Cu–N1a | 1.993(2) | N1a–Cu–N2a | 81.2(1) | N–Cu–O(an1) | 85.6–101.5(1) |
| Cu–N2a | 2.015(2) | N1b–Cu–N2b | 89.5(1) | N–Cu–O(an2) | 75.3–93.2(1) | |
| Cu–N1b | 2.003(2) | N1a–Cu–N1b | 175.6(1) | O(an1)–Cu–O(an2) | 176.7(1) | |
| Cu–N2b | 2.017(2) | N1a–Cu–N2b | 94.0(1) | |||
| Cu–O(an1) | 2.352(2) | N2a–Cu–N1b | 96.2(1) | |||
| Cu–O(an2) | 2.790(3) | N2a–Cu–N2b | 162.8(1) | |||
| 5a | Cu–N1a | 1.986(9) | N1a–Cu–N2a | 81.5(4) | N–Cu–O(an1) | 87.7–91.9(3) |
| Cu–N2a | 2.001(9) | N1a–Cu–N1b | 96.7(4) | N–Cu–O(an2) | 89.9–93.2(3) | |
| Cu–N1b | 1.999(8) | N2a–Cu–N1b | 178.2(4) | O(an1)–Cu–O(an2) | 177.6(3) | |
| Cu–O(w) | 1.931(8) | N1a–Cu–O(w) | 173.8(4) | O(w)–Cu–O(an1) | 92.2(3) | |
| Cu–O(an1) | 2.456(6) | N2a–Cu–O(w) | 92.3(4) | O(w)–Cu–O(an2) | 86.1(3) | |
| Cu–O(an2) | 2.488(7) | N1b–Cu–O(w) | 89.3(3) | |||
| 2b | Cu–N1a | 1.967(9) | N1a'–Cu–N1a | 80.7(6) | N1a–Cu–N1b' | 172.0(3) |
| Cu–N1b | 2.011(7) | N1b'–Cu–N1b | 88.1(4) | N–Cu–F | 82.1–96.9(3) | |
| Cu–F | 2.475(7) | N1a–Cu–N1b | 96.0(4) | |||
| 3b | Cu–N1a | 1.999(8) | N1a'–Cu–N1a | 81.1(5) | N1a–Cu–N1b' | 171.3(3) |
| Cu–N1b | 2.011(7) | N1b'–Cu–N1b | 90.3(4) | N–Cu–O(solv) | 89.6–97.5(3) | |
| Cu–O(solv) | 2.319(11) | N1a–Cu–N1b | 93.8(3) | |||
| Cu⋯F | 2.867(8) | |||||
| 4b | Cu–N1a | 1.970(12) | N1a–Cu–N2a | 81.4(5) | N–Cu–F | 82.3–96.4(4) |
| Cu–N2a | 1.998(11) | N1a–Cu–N1b | 95.4(4) | O(w)–Cu–F | 88.8–92.3(4) | |
| Cu–N1b | 2.013(10) | N2a–Cu–N1b | 176.5(5) | F–Cu–F | 168.6(3) | |
| Cu–O(w) | 1.992(8) | N1a–Cu–O(w) | 173.1(4) | |||
| Cu–F(an1) | 2.439(9) | N2a–Cu–O(w) | 93.8(4) | |||
| Cu–F(an2) | 2.532(10) | O(w)–Cu–N1b | 89.2(4) | |||
| 5b | Cu–N1a | 2.003(2) | N1a–Cu–N2a | 81.4(1) | N1b–Cu–Cl | 88.3(1) |
| Cu–N2a | 1.995(2) | N1a–Cu–N1b | 164.6(1) | N–Cu–Cl' | 94.0–99.8(1) | |
| Cu–N1b | 1.992(2) | N2a–Cu–N1b | 93.0(1) | Cl–Cu–Cl' | 92.36(2) | |
| Cu–Cl | 2.282(1) | N1a–Cu–Cl | 95.6(1) | |||
| Cu–Cl' | 2.651(1) | N2a–Cu–Cl | 173.2(1) | |||
| 2c | Cu–N1a | 1.983(10) | N1a–Cu–N2a | 80.1(5) | ||
| Cu–N2a | 2.003(10) | N1b–Cu–N2b | 88.2(7) | |||
| Cu–N1b | 2.014(8) | N1b–Cu–N2bb | 91.6(6) | |||
| Cu–N2b | 2.070(15) | O(w)–Cu–N | 85.4–102.0(4) | |||
| Cu–N1bb | 2.044(15) | O(w)–Cu–O(an) | 167.2(3) | |||
| Cu–N2bb | 2.037(8) | |||||
| Cu–O(w) | 2.307(10) | |||||
| Cu–O(an) | 2.793(11) | |||||
| 3c | Cu–N1a | 1.988(5) | N1a–Cu–N2a | 81.0(2) | N2a–Cu–O(an) | 93.0(2) |
| Cu–N2a | 2.014(5) | N1b–Cu–O(an) | 88.6(2) | N1a–Cu–O(w) | 98.1(2) | |
| Cu–N1b | 2.015(5) | N1a–Cu–N1b | 95.3(2) | N2a–Cu–O(w) | 92.7(2) | |
| Cu–O(an) | 1.991(4) | N2a–Cu–N1b | 167.2(2) | N1b–Cu–O(w) | 99.9(2) | |
| Cu–O(w) | 2.185(4) | N1a–Cu–O(an) | 168.8(2) | O(w)–Cu–O(an) | 91.6(2) | |
 | ||
| Fig. 1 A comparative view of the zig-zag chains, with the periods given on the left (Å) and the ‘stretching factor’ on the right (see text). | ||
While 2a, 2b and 3c show chains that are only slightly compressed, in the two derivatives of azpy a remarkable difference is observed, with higher compression in 3a and small elongation in 2c.
A view of the five polymers down their extension directions is reported in Fig. 2, showing the moderate rotations of the 2,2′-bipy ligands with respect to the plane of the Cu atoms.
 | ||
| Fig. 2 A view of the zig-zag chains along the running direction, showing the coordinations around the metals. | ||
In compound 2a the metal ions exhibit a square pyramidal five-coordination, with four equatorial N atoms (two from 2,2′-bipy and two from two different 4,4′-bipy ligands) and an axial interaction with an oxygen atom of a triflate. The second (disordered) anion is located nearly trans to the coordinated one, but the shortest Cu⋯O contact is too long (2.96 Å) and can be considered only as a weak secondary interaction. The rings of 4,4′-bipy are coplanar and the dihedral angle CuN2(2,2′-bipy)/CuN(4,4′-bipy)N(4,4′-bipy) is 13.5°. The Cu⋯Cu contacts for adjacent metals in the chains are 11.05 Å long. All the chains are parallel and extended in the [0 1 0] direction, with a period corresponding to the b crystallographic axis (13.42 Å). No particular interaction among the different chains is observed.
Compound 3a shows a distorted octahedral coordination geometry for the Cu(II) ions, with four equatorial N atoms (two of 2,2′-bipy and two of two different azpy ligands), and a water molecule (Cu–O 2.36 Å) and an oxygen atom of a triflate (Cu–O 2.62 Å) in the axial directions. The dihedral angle CuN2(2,2′-bipy)/CuN(azpy)N(azpy) is 7.6°. The Cu⋯Cu adjacent contacts in the chains are 12.89 Å. The chains (see Fig. 1) run in the [0 0 1] direction, with a period of the polymer equal to c (13.59 Å), which is similar to that of 2a in spite of the increased length of the spacer ligand (stretching factor 74%). The crystal structure shows an extended system of hydrogen bonds involving the coordinate H2O molecule and the triflate anions. Each water molecule forms two H-bonds: one with a free anion and the second with a coordinated triflate belonging to an adjacent chain. The latter bridges generate two-dimensional undulated layers of (4,4) topology, illustrated in Fig. 3.
![The packing down [0
1 0] of the chains in compound 3a
illustrating the 2D undulated layer joined by hydrogen bond bridges [O⋯O
2.731(6)
Å].](/image/article/2000/CE/b006306l/b006306l-f3.gif) | ||
| Fig. 3 The packing down [0 1 0] of the chains in compound 3a illustrating the 2D undulated layer joined by hydrogen bond bridges [O⋯O 2.731(6) Å]. | ||
We have observed also that the corresponding complex containing the bpethe ligand is isomorphous with the azpy species (3a).15
The coordination sphere of copper(II) in compound 2b exhibits four equatorial Cu–N bonds (with 2,2′-bipy and two bpethe ligands) and two weak axial Cu–F interactions with (disordered) BF4− anions. The dihedral angle CuN2(2,2′-bipy)/CuN(bpethe)N(bpethe) is 10.5°. The adjacent Cu⋯Cu contacts in a chain are 13.25 Å and all the chains (illustrated in Fig. 1) run in the [0 1 0] direction, with a period of the polymer equal to b (15.90 Å). The Cu–(BF4)–Cu bridges join the chains to give two-dimensional layers of (4,4) topology (see Fig. 4).
![The packing down [1
0 0] of the chains in compound 2b
showing the 2D layer bridged by the anions.](/image/article/2000/CE/b006306l/b006306l-f4.gif) | ||
| Fig. 4 The packing down [1 0 0] of the chains in compound 2b showing the 2D layer bridged by the anions. | ||
Large interchain voids (35% of the cell volume) are present, containing highly disordered solvent molecules, that were not included in the refined model. These channels run along [0 0 1] and contain also the free BF4− anions (see Fig. 5).
![The packing down [0
0 1] in compound 2b showing
the empty channels.](/image/article/2000/CE/b006306l/b006306l-f5.gif) | ||
| Fig. 5 The packing down [0 0 1] in compound 2b showing the empty channels. | ||
In compound 2c the coordination of Cu(II) is comprised of four equatorial Cu–N bonds (with 2,2′-bipy and the azpy spacers), an axial Cu–O(H2O) bond (2.31 Å) and a weak axial interaction with a nitrate, Cu–O (2.79 Å). The dihedral angle CuN2(2,2′-bipy)/CuN(azpy)N(azpy) has the values 13.0 and 20.8° for the two equal weight models used for the disordered ligand. The Cu⋯Cu contacts in each chain are 13.02 Å, only slightly longer than in 3a, while the period of the polymer is significantly longer than in 3a, 19.07 Å (see Fig. 1). In contrast to the other zig-zag polymers here described, in which all the chains run parallel, this species exhibits two distinct directions of propagation for the chains, along [1 1 0] and [1 −1 0]. The two sets of chains show a quite uncommon type of supramolecular entanglement, generating warp-and-woof like two-dimensional sheets, illustrated in Fig. 6.
 | ||
| Fig. 6 The sphere packing (top) and the schematic view (bottom) of the warp-and-woof sheet in compound 2c. | ||
This is, to our knowledge, the first example of this type of supramolecular organization for one-dimensional coordination polymers. Similar entangled layers were previously observed only in [(AuI)2(μ-bis(diphenylphosphino)hexane)], containing dinuclear units that are joined into infinite chains only thanks to weak aurophilic Au⋯Au contacts [3.124(2) Å long].16 Few other types of unusual entanglements of one-dimensional polymers are known at present: we have already reported two of these rare cases, namely an infinite double helix17 and a three-dimensional array derived by chains running in three not coplanar directions.18 The warp-and-woof layers are superimposed along the [0 0 1] direction with an ABAB sequence, and each layer is interdigitated with the two adjacent (upper and lower) ones, giving π–π stacking interactions involving the 2.2′-bipy ligands. This generates a three-dimensional extended array (see Fig. 7).
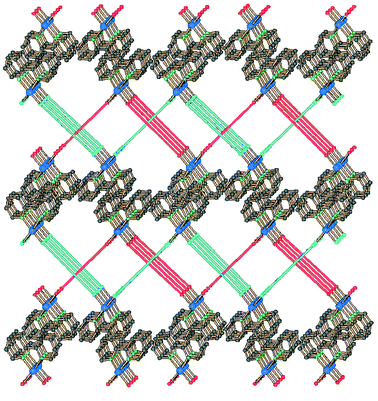 | ||
| Fig. 7 The stacking of the 2D layers of compound 2c showing the π–π interactions involving the 2,2′-bipy ligands (stacking distance 3.41 Å). | ||
The last isolated species of this class is compound 3c. In this polymer the bridging ligand is the i-nicotinate anion, that uses the N atom of the 4-pyridyl group and one oxygen atom of the carboxylate group as donors. The metal ions exhibit a square pyramidal coordination, with three N and one O equatorial atoms (two N from 2,2′-bipy, one N from the 4-pyridyl group of i-nicotinate and one O from the monodentate carboxylate group of another i-nicotinate), and an axial interaction with a coordinated water molecule. The dihedral angle CuN2(2,2′-bipy)/CuN(i-nic)O(i-nic) is 15.4°. The Cu⋯Cu contacts for adjacent metals in the chains are 8.88 Å long. The chains (see Fig. 1) are all parallel and extended in the [0 1 0] direction, with a period corresponding to the b axis (11.55 Å). An extended system of hydrogen bonds is present, involving all the water molecules (coordinated and solvated) and both the nitrate and the i-nicotinate anions (see Fig. 8).
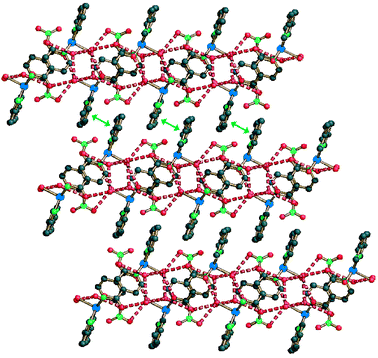 | ||
| Fig. 8 A lateral view of the 2D layers in compound 3c showing the extended hydrogen bond system involving solvated water molecules (dashed red lines, O⋯O range 2.83–2.89 Å). The stacking of the layers shows interdigitation and π–π interactions (green double arrows, stacking separation 3.54 Å). | ||
The festoon polymer 3b
A different type of one-dimensional polymer is present in 3b, assembled with the flexible ligand bpp. This ligand adopts the trans–trans (TT) conformation and the chain appears as a festoon, as shown in Fig. 9. The Cu(II) coordination geometry is comprised of four equatorial N atoms (two N of 2,2′-bipy and two N of different bpp), and an axial oxygen atom of a coordinated ethanol molecule. In the trans axial direction a very weak interaction with one BF4− anion is observed (Cu⋯F 2.87 Å). The dihedral angle CuN2(2,2'-bipy)/CuN(bpp)N(bpp) is 10.1°. The Cu⋯Cu contact for adjacent metals is 11.96 Å, the same value, in this case, as the period of the polymers, that extend in the [0 1 0] direction. Some selected bond distances and angles are given in Table 3. | ||
| Fig. 9 A view of the festoon polymer in compound 3b. | ||
Discrete molecular architectures
Few examples of discrete molecular species have also been obtained from 1. In these compounds (4a, 5a and 4b) the exo-bidentate ligands do not give polymerization, though in two of them other interactions (via the anions or H-bonds) produce extended systems.Compound 4a consists of molecular rings in which two flexible bpetha ligands in the gauche conformation join two Cu(II) units (see Fig. 10). The metal coordination sphere is completed by two triflate anions in the axial direction, that display quite different interactions with the copper ion (Cu–O 2.35 and 2.79 Å). The whole 22-membered ring lies about a crystallographic inversion centre. The planes of the aromatic rings of the bpetha ligands are nearly perpendicular to the average plane of the macrocycle. The dihedral angle CuN2(2,2′-bipy)/CuN(bpetha)N(bpetha) is 6.6°. The Cu⋯Cu trans-annular contact is 9.99 Å, while the methylenic carbons of the bpetha ligands show contacts in the range 8.93–9.14 Å. A sphere packing view showing the inner cavity is shown in Fig. 10 (bottom). The intermolecular interactions are essentially of the van der Waals type.
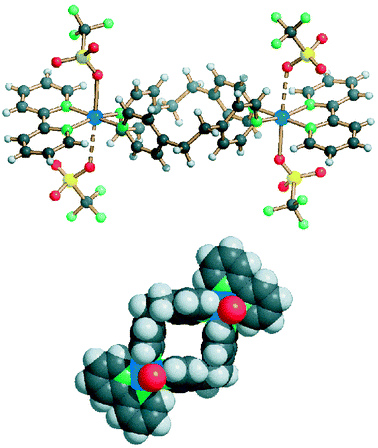 | ||
| Fig. 10 A lateral view (top) and the sphere packing (bottom, showing only the oxygen atoms of the coordinated anions) for compound 4a. Click image or Fig10.htm to access 3D representation. | ||
Many examples of similar dimetallic molecular rings have been previously reported.3,4 However, the presence in 4a of ‘octahedral’ metal units makes it rather uncommon, since the majority of the other species contains square planar cis-Pd(II) or cis-Pt(II) units. A molecular ring assembled with the same bpetha ligand and [(en)Pd(NO3)2] has been reported by Fujita et al.6c
Compound 5a contains centrosymmetric dinuclear complexes, shown in Fig. 11 (left). One pyz ligand connects two units 1, in which the fourth equatorial positions are occupied by coordinated water molecules.
 | ||
| Fig. 11 The dinuclear complex of 5a (left) and the molecular ladder formed by the bridging triflates (right). Click image or Fig11.htm to access 3D representation. | ||
The planes of the two 2,2′-bipy ligands are not coplanar (dihedral angle 21.2°) and are rotated by equal amounts (dihedral angle 79.4°) on opposite sites with respect to the plane of the central pyz ligand. The two axial positions of the copper ions are occupied by oxygen atoms of triflate anions (Cu–O 2.49 and 2.46 Å), that act in a μ–η2-bridging mode. These interactions generate a ladder like infinite motif (Fig. 11, right), in which the dinuclear moieties are the rungs (Cu⋯Cu 6.82 Å) and the Cu–(triflate)–Cu bridges the siderails (Cu⋯Cu 7.24 Å). The coordinated water molecules form hydrogen bonds with the free triflate anions. All the ladders have their planes parallel and run along the direction of the c axis. Channels are observed around the ladders, extending in the same direction (see Fig. 12). The free voids correspond to 18% of the cell volume.
 | ||
| Fig. 12 A view down c of the packing in compound 5a showing the empty channels left around the ladders. The central ladders are shifted along c by a half of a siderail with respect to the lateral ones. | ||
Compound 4b is a mononuclear complex (Fig. 13, top). The two free equatorial positions of 1 are occupied by a water molecule and a monodentate 2,4′-bipy, that donates with the N atom of the 4-pyridyl group. The two BF4− anions give interactions in the axial direction (Cu–F 2.43 and 2.52 Å). The 2,4′-bipy ligand is able to act as exo-bidentate with digonal metals as Ag(I), generating chains.19 With 1, therefore, the only suitable coordination positions could be the two trans axial, but the weakness of the interactions along these directions leads to a different product. Noteworthy the mononuclear complexes are joined into one-dimensional chains (see Fig. 13, bottom) via OH⋯N hydrogen bond bridges involving the coordinated water molecules and the 2-pyridyl groups on adjacent complexes.
![A view of the complex 4b (top) and the chain formed by hydrogen
bond bridges [bottom, O⋯N 2.735(13) Å].
The planes of the rings of the 2,4'-bipy ligand are rotated, with
a dihedral angle of 28.8(5)°, to favour the hydrogen bonds.](/image/article/2000/CE/b006306l/b006306l-f13.gif) | ||
| Fig. 13 A view of the complex 4b (top) and the chain formed by hydrogen bond bridges [bottom, O⋯N 2.735(13) Å]. The planes of the rings of the 2,4'-bipy ligand are rotated, with a dihedral angle of 28.8(5)°, to favour the hydrogen bonds. | ||
A polymeric chain of dinuclear units (5b)
Compound 5b is rather different from the other species here described since it contains one chlorine atom per copper atom. The basic moiety in this case is the dinuclear centrosymmetric species [(2,2′-bipy)Cu(μ-Cl)2Cu(2,2′-bipy)]2+, in which two units 1 are asymmetrically bridged by two chlorides (see Fig. 14). The coordination of the Cu(II) ions is square pyramidal, involving the 2,2′-bipy, the N atom of a bridging dinitrile and a chloride ion in equatorial position, and exhibiting a weaker Cu–Cl interaction (2.65 vs. 2.28 Å) in the axial direction. | ||
| Fig. 14 A view of the dinuclear unit in complex 5b, showing also the terminal N atoms of the dinitrile ligands. Click image or Fig14.htm to access 3D representation. | ||
The hdn ligands adopt the GTTG conformation and link the dinuclear units into winding chains (Fig. 15), all extending in the direction of the c crystallographic axis (with a period equal to c, 21.25 Å). The 2,2′-bipy rings on adjacent chains give π–π stacking interactions that extend the array into two-dimensional layers (see Fig. 16).
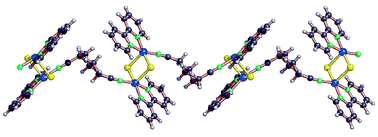 | ||
| Fig. 15 A view of the polymeric chain in compound 5b. | ||
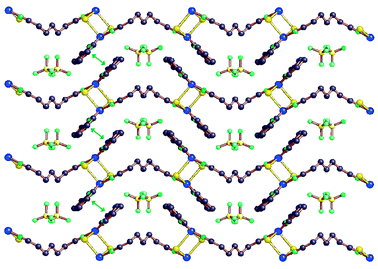 | ||
| Fig. 16 A 2D layer formed by the π–π stacking interactions (green double arrows, stacking separation 3.44 Å) among the chains in 5b. | ||
Conclusions
The results here reported illustrate the possibility of using 1 as a ‘90° corner’ building block. Only few of the motifs of Scheme 1 have been, at present, isolated (I, IV and V), but we are performing a systematic screening of the reaction conditions in order to obtain other molecular architectures,20 like the hypothetical structure illustrated in Scheme 1. However, rather than the achievement of a particular interesting product, the main goal of our research is to reach a better understanding of the factors that can control the self-organization of these systems, and the present findings can be considered only as the result of a preliminary approach to the problem. Nevertheless, some of the structural motifs illustrated above are particularly noteworthy per se, as the warp-and-woof layers in 2c and the molecular rings in 4a.References
- (a) B. F. Hoskins and R. Robson, J. Am. Chem. Soc., 1990, 112, 1546 CrossRef CAS; (b) R. Robson, B. F. Abrahams, S. R. Batten, R. W. Gable, B. F. Hoskins and J. Liu, in Supramolecular Architecture, ACS, Washington, DC, 1992, ch. 19. Search PubMed; (c) M. J. Zaworotko, Chem. Soc. Rev., 1994, 283 RSC; (d) C. L. Bowes and G. A. Ozin, Adv. Mater., 1996, 8, 13 CrossRef CAS; (e) O. M. Yaghi, H. Li, C. Davis, D. Richardson and T. L. Groy, Acc. Chem. Res., 1998, 31, 474 CrossRef CAS; (f) M. Munakata, L. P. Wu and T. Kuroda-Sowa, Adv. Inorg. Chem., 1999, 46, 173 CrossRef; (g) P. J. Hagrman, D. Hagrman and J. Zubieta, Angew. Chem., Int. Ed., 1999, 38, 2638 CrossRef; (h) A. J. Blake, N. R. Champness, P. Hubberstey, W. S. Li, M. A. Withersby and M. Schröder, Coord. Chem. Rev., 1999, 183, 117 CrossRef CAS.
- Comprehensive Supramolecular Chemistry, ed. J.-M. Lehn, Pergamon Press, Oxford, 1995. Search PubMed.
- (a) M. Fujita and K. Ogura, Coord. Chem. Rev., 1996, 148, 249 CrossRef CAS; (b) M. Fujita, Acc. Chem. Res., 1999, 32, 53 and refs. therein. Search PubMed.
- (a) P. J. Stang, Chem. Eur. J., 1998, 4, 19 CrossRef CAS; (b) S. Leininger, B. Olenyuk and P. J. Stang, Chem. Rev., 2000, 100, 853 and refs. therein. Search PubMed.
- R.-D. Schnebeck, E. Freisinger, F. Glahé and B. Lippert, J. Am. Chem. Soc., 2000, 122, 1381 CrossRef CAS.
- (a) M. Fujita, J. Yazaki and K. Ogura, J. Am. Chem. Soc., 1990, 112, 5645 CrossRef CAS; (b) M. Fujita, J. Yazaki, T. Kuramochi and K. Ogura, Bull. Chem. Soc. Jpn., 1993, 66, 1837 CrossRef CAS; (c) M. Fujita, S. Nagao, M. Iida, K. Ogata and K. Ogura, J. Am. Chem. Soc., 1993, 115, 1574 CrossRef CAS; (d) M. Fujita, M. Aoyagi and K. Ogura, Inorg. Chim. Acta, 1996, 246, 53 CrossRef CAS; (e) M. Fujita, O. Sasaki, T. Mitsuhashi, T. Fujita, J. Yazaki, K. Yamaguchi and K. Ogura, Chem. Commun., 1996, 1535 RSC.
- K. Hyde, G. F. Kokoszka and G. Gordon, J. Inorg. Nucl. Chem., 1969, 31, 1993 CrossRef CAS.
- G. M. Sheldrick, SADABS: Siemens Area Detector Absorption Correction Software, University of Göttingen, 1996..
- A. Altomare, M. C. Burla, M. Camalli, G. Cascarano, C. Giacovazzo, A. Guagliardi, A. G. Moliterni, G. Polidori and R. Spagna, J. Appl. Crystallogr., 1999, 32, 115 CrossRef CAS.
- G. M. Sheldrick, SHELX-97, University of Göttingen, 1997..
- A. L. Speck, PLATON, A Multipurpose Crystallographic Tool, Utrecht University, 1999. An analysis of the holes was performed with this program..
- E. Keller, SCHAKAL99, University of Freiburg, 1999..
- M. T. Garland, D. Grandjean, E. Spodine, A. M. Atria and J. Manzur, Acta Crystallogr., Sect. C, 1988, C44, 1209 CrossRef CAS.
- Complex [Cu(2,2′-bipy)(H2O)2(CF3SO3)2] (1a) is monoclinic, C2/c, Z = 4, a = 9.506(2), b = 18.389(6), c = 12.169(3) Å, β = 101.62(2)°, V = 2083(1) Å3. Complex [Cu(2,2′-bipy) (H2O)2(BF4)2] (1b) is monoclinic, C2/c, Z = 4, a = 16.819(13), b = 12.665(6), c = 7.137(12) Å, β = 91.67(9)°, V = 1520(3) Å3. L. Carlucci, G. Ciani, D. M. Proserpio and S. Rizzato, results to be published..
- Complex [(1)(bpethe)(H2O)(CF3SO3)](CF3SO3) is triclinic, P
![[1 with combining macron]](https://www.rsc.org/images/entities/char_0031_0304.gif) , a = 9.003(5), b = 11.959(3), c = 13.656(4) Å, α = 88.85(2), β = 87.78(3), γ = 87.33(3)°, V = 1467(1) Å3..
, a = 9.003(5), b = 11.959(3), c = 13.656(4) Å, α = 88.85(2), β = 87.78(3), γ = 87.33(3)°, V = 1467(1) Å3.. - P. M. Van Calcar, M. M. Olmestead and A. L. Balch, J. Chem. Soc., Chem. Commun., 1995, 1773 RSC.
- L. Carlucci, G. Ciani, D. W. v. Gudenberg and D. M. Proserpio, Inorg. Chem., 1997, 36, 3812 CrossRef CAS.
- L. Carlucci, G. Ciani, P. Macchi, D. M. Proserpio and S. Rizzato, Chem. Eur. J., 1999, 5, 237 CrossRef CAS.
- M.-L. Tong, X.-M. Chen, B.-H. Ye and S. Weng Ng, Inorg. Chem., 1998, 37, 5278 CrossRef CAS.
- We have some preliminary evidences of the formation of a molecular square (structure II of Scheme 1) using the bpetha ligand, that will be reported elsewhere..
| This journal is © The Royal Society of Chemistry 2000 |
