Generation of lanthanide coordination polymers with dicarboxylate ligands: synthesis, structure, thermal decomposition and magnetic properties of the two-dimensional triaquatris(malonato)dipraseodymium(III) dihydrate {[Pr2(C3H2O4)3(H2O)3]·2H2O}
María Hernández-Molinaa, Pablo A. Lorenzo-Luisb, Trinidad Lópeza, Catalina Ruiz-Pérez*c, Francesc Lloretd and Miguel Julved
aGrupo de Crecimiento Cristalino, Departamento de Física Básica, Universidad
de La Laguna, Avda. Astrofísico
Francisco Sánchez s/n, 38204, La
Laguna, Tenerife, Spain
bDepartamento de Química Inorgánica, Universidad de La Laguna, 38204, La Laguna, Tenerife, Spain
cDepartamento de Física Fundamental
II, Universidad de La Laguna, Avda. Astrofísico Francisco Sánchez s/n, 38204, La Laguna, Tenerife, Spain. E-mail: caruiz@ull.es
dDepartament de Química Inorgànica, Facultat de Química, Universitat de València, Avda. Dr. Moliner 50, 46100, Burjassot (València), Spain
Abstract
The malonate complex of formula [Pr2(C3H2O4)3(H2O)3]·2H2O (1) was prepared and his crystal structure determined by X-ray diffraction methods. 1 crystallizes in the monoclinic space group P21, Z = 4, with unit cell parameters a = 7.631(2), b = 12.899(4), c = 8.923(2) Å and β = 101.11(3)°. 1 is a polymer which grows in the (110) plane. The hydrogen bond stabilizes the crystal structure forming a three-dimensional network. The two non-equivalent praseodymium(III) ions have different environments. Finally, the thermal behaviour and magnetic properties were investigated.
Introduction
The design of new solid-state architectures has recently driven much attention and increasing interest.1 Advanced crystal engineering by selecting structural ligands and the coordination geometry of lanthanide(III) metal centers as a building block can give a series of novel inorganic frameworks with an interesting variety of crystal-packing motifs. This is in accordance with the experience that the crystal structures of the lanthanide compounds frequently change along the series as a result of the lanthanide contraction.Among a number of ligands used as building blocks, the two end functional groups of dicarboxylic acids could yield a variety of crystal structures of coordination compounds even with close chemical formulae, depending on the conformation of the carbon chains and the end functional groups. Those compounds are characterized as organic/inorganic hybrids with pillared sheet-like structures assembled from the molecular precursor in solution.
In order to check the ability of rare earth ions to lead to high dimensional materials, we have thus undertaken a study of the lanthanide malonate complexes (where malonate stands for the dianion of the propanedioic acid, H2mal). This ligand has, indeed, a great ability to form infinite connections with metal ions2,3 and a remarkable versatility in adopting several different modes of bonding, ranging from unidentate, chelating and bridging, sometimes in more than one way in the same compound. Seven bonding modes have been observed according to the coordination environment of COO− groups as shown in Scheme 1.
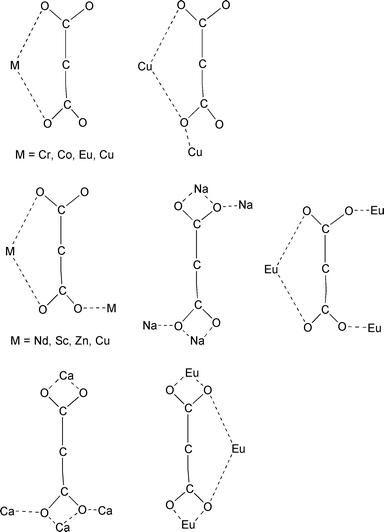 | ||
| Scheme 1 | ||
Also, malonic acid is widely used in many important fields as a result of its excellent coordination ability. The complexes of malonic acid with lanthanide and transition elements have been used in the fluorescent probe technique, solid fluorescent materials, biological active materials and modifiers of polyethylene.4,5 Therefore, studies of the new complexes with malonic acid are quite valuable in theory and application.
In this paper we describe the synthesis, IR spectrum, thermal behaviour, magnetic properties and crystal structure of [Pr2(mal)3(H2O)3]·2H2O, (1).
Experimental
Materials
Malonic acid, praseodymium(III) nitrate hexahydrate Pr(NO3)3·6H2O and sodium metasilicate nonahydrate Na2Si O3·9H2O were purchased from commercial sources and used as received. Elemental analysis (C,H) was performed with an EA 1108 CHNS-0 automatic analyzer.Physical techniques
Thermogravimetric (TG) and calorimetric analyses were performed on a Netzsch STA 409 EP simultaneous thermobalance and a differential scanning calorimeter (DSC), respectively, at 5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1
to a maximum temperature of 400
°C min−1
to a maximum temperature of 400![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C in dynamic dinitrogen atmosphere
of ca. 70 cm3 min−1. Variable-temperature (1.9–290 K)
magnetic susceptibility measurement on a polycrystalline sample was carried
out with a Quantum Design SQUID magnetometer operating at 100 G (T < 50 K)
and 1000 G (over the whole range).
°C in dynamic dinitrogen atmosphere
of ca. 70 cm3 min−1. Variable-temperature (1.9–290 K)
magnetic susceptibility measurement on a polycrystalline sample was carried
out with a Quantum Design SQUID magnetometer operating at 100 G (T < 50 K)
and 1000 G (over the whole range).Synthesis
Single crystals have been grown in silica gel medium, using the techniques described by Henisch.6 An approximately 1 M solution of sodium metasilicate nonahydrate was added to a volume of 2 M malonic acid with continuos stirring. The mixture was introduced into test tubes, covered, and allowed to set for one day at room temperature. A 0.5 M solution of Pr(NO3)3·6H2O was introduced above the gel, care being taken to avoid damaging the surface of the gel, and the tubes were stored at 40![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C. Three weeks later,
green crystals appeared suitable for X-ray analysis. Anal. Calc. for C9H16O17Pr2:
C, 15.94; H, 2.38. Found: C, 17.49; H, 2.56%.
°C. Three weeks later,
green crystals appeared suitable for X-ray analysis. Anal. Calc. for C9H16O17Pr2:
C, 15.94; H, 2.38. Found: C, 17.49; H, 2.56%.Crystallographic data collection and structure determination
A crystal of dimensions 0.45 × 0.60 × 0.24 mm3 was used for data collection on an Enraf–Nonius MACH3 four-circle diffractometer. Data were collected at 293(2) K using graphite-monochromatized MoKα radiation (λ = 0.71069 Å) and the ω-scan technique. Details of the crystal structure are given in Table 1. The data were corrected for Lorentz, polarization and absorption.9 The maximum and minimum transmission factors were 1.693 and 0.476. The structure was solved using SIR9710 and refined using SHELXL97.11 In the final least squares cycles all non-H atoms were allowed to vibrate anisotropically. Hydrogen atoms of the malonate ligand were included at calculated positions on carbon centres. Hydrogen atoms attached to oxygen atoms of water molecules were not found. The final geometrical calculations and the graphical manipulations were carried out with the PARST9512 and PLATON13 programs, respectively.| Chemical formula | C9H16O17Pr2 |
|---|---|
| a Click here for full crystallographic data (CCDC no. 1350/37). | |
| Formula weight | 678.03 |
| T/K | 293(2) |
| Crystal system | Monoclinic |
| Space group | P21 |
| a/Å | 7.631(2) |
| b/Å | 12.899(4) |
| c/Å | 8.923(2) |
| β/° | 101.11(3) |
| V/Å3 | 861.9(4) |
| Dc/g cm−3 | 2.613 |
| Z | 4 |
| µ/mm−1 | 5.678 |
| Measured/independent reflections R(int) | 5372/4962 0.012 |
| Final R indices [I > 2σ(I)] | R1 = 0.031, wR2 = 0.081 |
| R indices (all data) | R1 = 0.032, wR2 = 0.082 |
Results and discussion
Description of the structure
The structure of the compound can be visualised as chains of praseodymium(III) ions linked through two crystallographically independent malonate ligands, L1 and L2 (see Fig. 1a), which run parallel to the a axis. Their conformation is the same: L1 and L2 are three-times chelating including the six-membered rings, which adopts boot conformation. The O(1), O(3), O(6) and O(7) are the oxygen–carboxylate atoms chelate praseodymium ions and they also coordinate to adjacent praseodymium ions in the form of μ2–O. The distances Pr⋯Pr through this bridging pathway is 4.2301(10) Å [Pr2⋯Pr1(1 + x, y, z)] for O(1) 4.4605(9) Å (Pr1⋯Pr2) for O(3) and O(6) and 4.2301(10) Å [Pr1⋯Pr2(−1 + x, y, z)] for O(7).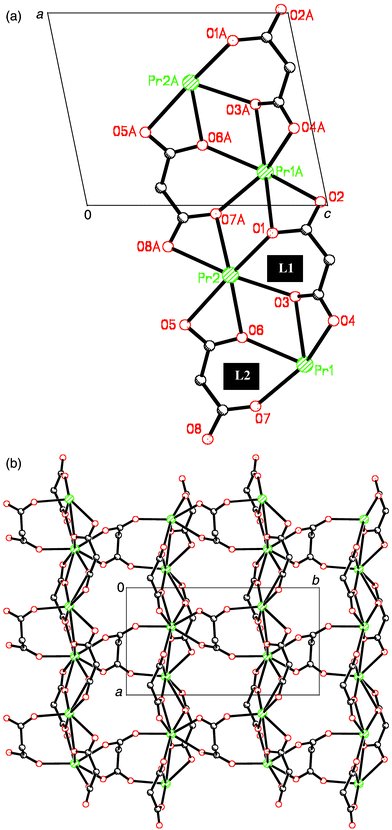 | ||
| Fig. 1 (a) Perspective view of the chain. (b) A view of a fragment of the layer in the (110) plane. | ||
The chains are linked in the perpendicular direction (along the crystallographic b axis) through a new independent malonate ligand, L3 (Fig. 1b), forming a two-dimensional structure. L3 adopts simultaneously bidentate [at Pr(2)] and monodentate [at Pr(1)] coordination modes and exhibits a boat conformation (see Fig. 2). Two carboxylate bridges O(9)C(7)O(10) and O(11)C(9)O(12) exhibit the anti–anti conformation. A hydrogen bond between one coordinated water molecule [O(2w)] and a carboxylate–oxygen atom O(9), O(2w)⋯O(9)(−1 + x, y, z) 3.151(7) Å occurs within each layer. Bidimensional networks can be found in different malonate complexes containing lanthanides14 and metal transitions ions.15–17
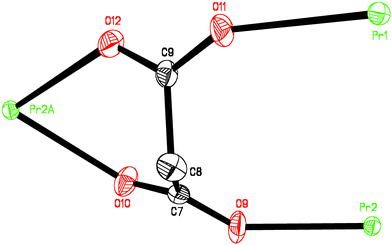 | ||
| Fig. 2 Malonate L3 conformation: the thermal ellipsoids are drawn at the 50% probability level and the hydrogen atoms have been omitted. Symmetry codes a = −1 − x, −½ + y, −1 − z. | ||
The crystal structure is stabilized through extensive hydrogen bonding involving carboxylate groups and water molecules, forming a three-dimensional network (see Fig. 3 and Table 2). In all hydrogen bonds, the water oxygens act as donor atoms, and the carboxylate or other water oxygens act as acceptor atoms. Furthermore, additional hydrogen bonds between a carboxyl group and a water molecule occur.
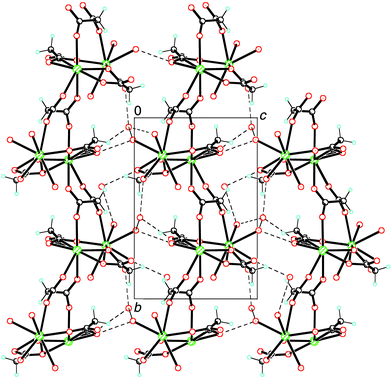 | ||
| Fig. 3 Projection of the structure down the a axis. Dashed lines correspond to hydrogen bridging. | ||
| D⋯A | D⋯A | ||
|---|---|---|---|
| Symmetry codes: a = x, y, z − 1; b = x, y, z + 1; c = x − 1, y, z − 1; d = −2 − x, y + ½ , −1 − z; e = −2 − x, −½ + y, −1 − z. | |||
| O1w⋯O2c | 2.820(7) | O4w⋯O4e | 2.858(7) |
| O1w⋯O11d | 2.976(7) | O4w⋯O8 | 2.729(7) |
| O1w⋯O12d | 3.006(8) | O4w⋯O2wa | 2.880(9) |
| O1w⋯O2wd | 2.852(8) | O4w⋯O3wc | 2.815(7) |
| O3w⋯O5b | 2.724(6) | O4w⋯H8A-C8c | 2.603(5) |
The two crystallographically independent praseodymium(III) ions [Pr(1) and Pr(2)] have different coordination numbers. Pr1 is coordinated by three water molecules and five malonate ligands. The coordination number of Pr1 is ten and the coordination geometry is a distorted bi-capped dodecahedron, while Pr2 is surrounded by nine oxygen atoms forming a distorted monocapped square antiprism. Both geometries are observed in other lanthanide–malonate complexes reported in the literature.18–20 The oxygens around Pr2 are provided by five malonate ligands. Both polyhedrons share two corners O(3) and O(6). The praseodymium ions environments are shown in Fig. 4a and the two polyhedrons are depicted in Fig. 4b. The Pr1–O bond distances are in the range 2.433(4)–2.719(5) Å; their average value is 2.573(4) Å. The bond Pr1–O1(−1 + x, y, z) is considerably longer than the average value. The Pr2–O bond distances are smaller and they lie in the range 2.393(5)–2.684(4) Å, with an average value of 2.526(4) Å. This value is similar to that found for nine-coordinated praseodymium cations.
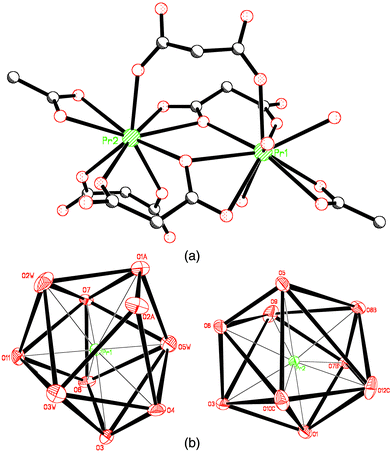 | ||
| Fig. 4 (a) Perspective drawing of praseodymium ions environments. (b) Coordination geometry of both praseodymium cations. | ||
The average C–O bond distances and O–C–O bond angles are 1.254(7) Å and 120.7(5)° for L1 and L2, and 123.3(5)° for L3, respectively. These values agree well with that of other previously reported malonate-containing lanthanide complexes.15–17
Thermal analyses
TG-DSC of [Pr2(mal)3(H2O)3]·2H2O 1 (Fig. 1) shows a first step with two mass loss. The first gradual mass loss is between 29 and 75![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C with an endothermic process centered at 48
°C with an endothermic process centered at 48![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C (5.14%).
The second mass loss occurs between 76 and 193
°C (5.14%).
The second mass loss occurs between 76 and 193![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C with two endothermic
processes—one centered at 85
°C with two endothermic
processes—one centered at 85![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C and the other at 152
°C and the other at 152![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C (8.02%).
The total observed weight loss in the latter two stages is 13.16% and
it corresponds to the release of five water molecules per formula unit (calculated
value is 13.23%). This process seems to take place in two steps,
suggesting that all water molecules are not similar, as deduced from the crystallographic
data (vide supra). The temperatures for weight loss of
hydration and coordinated water molecules are in agreement with those found
for other compounds described in the literature.3,21–25
°C (8.02%).
The total observed weight loss in the latter two stages is 13.16% and
it corresponds to the release of five water molecules per formula unit (calculated
value is 13.23%). This process seems to take place in two steps,
suggesting that all water molecules are not similar, as deduced from the crystallographic
data (vide supra). The temperatures for weight loss of
hydration and coordinated water molecules are in agreement with those found
for other compounds described in the literature.3,21–25The second step of weight loss occurs between 193 and 300![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C
as shown in Fig. 1, and it is a strong
exothermic process [Tpeak]DSC = 291
°C
as shown in Fig. 1, and it is a strong
exothermic process [Tpeak]DSC = 291![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C,
which is caused by the combustion of part of organic matter of the organic
ligand.3,21)
°C,
which is caused by the combustion of part of organic matter of the organic
ligand.3,21)
![TG and DSC curves at
5 °C min−1 in dynamic dinitrogen atmosphere at
70 cm3 min−1 for [Pr2
(mal)3
(H2O)3]·2H2O (1).](/image/article/2000/CE/b006256l/b006256l-f5.gif) | ||
Fig. 5 TG and DSC curves at
5![[thin space (1/6-em)]](https://www.rsc.org/images/entities/char_2009.gif) °C min−1 in dynamic dinitrogen atmosphere at
70 cm3 min−1 for [Pr2
(mal)3
(H2O)3]·2H2O (1). °C min−1 in dynamic dinitrogen atmosphere at
70 cm3 min−1 for [Pr2
(mal)3
(H2O)3]·2H2O (1). | ||
Magnetic properties
The magnetic properties of 1 under the form of the χMT product vs.T [χM being the magnetic susceptibility per two Pr(III) cations] are shown in Fig. 6. The value of χMT at room temperature is approximately 2.80 cm3 mol−1 K and it continuously decreases upon cooling. An incipient maximum in the susceptibility curve (see insert of Fig. 6) is observed at ca. 20 K. These features are indicative of an overall antiferromagnetic coupling. The examination of the crystal structure of 1 reveals the presence of chains of Pr(III) cations which are bridged by two malonate–oxygen atoms. Consequently, the exchange pathway for the antiferromagnetic coupling observed most likely involves these oxygen atoms. The ground state for a Pr(III) single ion is 3H4 and the first excited state, 3H5, is 2000 cm−1 above the low-lying one. So, the ground state is the only thermally populated at room temperature and the magnetic properties of 1 are due to this term. Keeping in mind that the angular momentum J = 4 is large enough as to be considered a classical angular momentum and neglecting the ligand field effects, we can use the Fisher equation describing a Heisenberg classical magnetic chain. For that, we use S = 4 in the Fisher expression, the Hamiltonian being H = −j ΣiSi·Si + 1. The best-fit results are: j = −1.8 cm−1, g = 0.79, ρ = 0.03%, and R = 9.0 × 10−5ρ and R are the percentage of monomeric impurities and the agreement factor defined as Σi[(χM)obs(i) − (χM)calc(i)]2/Σi[(χM)obs(i)]2, respectively. The computed value of g is in good agreement with that expected for the ground state 3H4 (g = 0.80). The calculated curve (solid line in Fig. 6) matches very well the experimental magnetic data except in the very low temperature region. It is important to note that ligand field effects break the degeneration of the levels of the ground state multiplet. So, the calculated value for the exchange coupling parameter must be considered as an upper limit for the intrachain antiferromagnetic interaction. In fact, the influence of the ligand field effects can be seen in the very low temperature region where the theoretical model is unable to reproduce the experimental susceptibility data.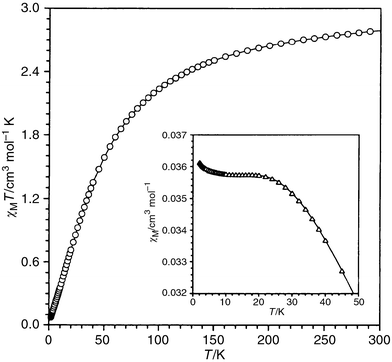 | ||
| Fig. 6 Thermal dependence of the χMT product. The inset shows the thermal dependence of the molar magnetic susceptibility. | ||
Acknowledgements
Financial support from Spanish Dirección General de Investigación Científica y Técnica (DGICYT) through Projects PB97-1479-C02-02 and PB97-1397, from the Gobierno Autónomo de Canarias through Project PI1999/061 and from the European Union (TMR Programme) through Contract ERBFM-RXCT980181 is gratefully acknowledged.References
- (a) G. B. Gardner, D. Venkataraman, J. S. Moore and S. Lee, Nature, 1995, 374, 792 CrossRef CAS; (b) B. F. Abrahams, B. F. Hoskins, A. M. Michall and R. Robson, Nature, 1994, 374, 727 CrossRef; (c) D. Braga, F. Grepioni and G. R. Desiraju, Chem. Rev., 1998, 98, 1375 CrossRef CAS PubMed; (d) J. Lu, C. Yu, T. Niu, T. Paliwala, G. Crisci, F. Somosa and A. J. Jacobson, Inorg. Chem., 1998, 37, 4637 CrossRef CAS PubMed; (e) M. L. Tong, S. L. Zheng and X. M. Chen, Chem. Commun., 1999, 561 RSC; (f) G. Alberti, M. Casuola, V. Constantino and R. Vivani, Adv. Mater., 1996, 8, 291 CrossRef CAS.
- (a) N. J. Ray and B. J. Hathaway, Acta Crystallogr., Sect. B, 1982, 38, 770 CrossRef; (b) R. Hämäläinen and A. Pajunen, Suom. Kemistil. B, 1973, 46, 285 Search PubMed; (c) W. L. Kwik, K. P. Ang, S. O. Chan; V. Chebolu and S. A. Koch, J. Chem. Soc., Dalton Trans., 1986, 2519 RSC; (d) D. Chattopadhyay, S. K. Chattopadhyay, P. R. Lowe, C. H. Schawalbe, S. K. Mazumber, A. Rana and S. Ghosh, J. Chem. Soc., Dalton Trans., 1993, 913 RSC; (e) A. Tosik, L. Sieron and M. Bukowska-Strzyzewska, Acta Crystallogr., Sect. C, 1995, 51, 1987 CrossRef; (f) E. Suresh and M. H. Bhadbhade, Acta Crystallogr., Sect. C, 1997, 53, 193 CrossRef; (g) C. Ruiz-Pérez, J. Sanchiz, M. Hernández-Molina, F. Lloret and M. Julve, Inorg. Chem., 2000, 39, 1363 CrossRef; (h) C. Ruiz-Pérez, M. Hernández-Molina, P. Lorenzo-Luis, F. Lloret, J. Cano and M. Julve, Inorg. Chem., 2000, accepted Search PubMed; (i) Y. Rodríguez-Martín, C. Ruiz-Pérez, J. Sanchiz, F. Lloret and M. Julve, Inorg. Chem., 2000, accepted Search PubMed.
- (a) I. Gil de Muro, F. A. Mautner, M. Insausti, L. Lezama, J. L. Pizarro, M. L. Arriortua and T. Rojo, Eur. J. Inorg. Chem., 1999, 935 CrossRef; (b) C. Ruiz-Pérez, J. Sanchiz, M. Hernández-Molina, F. Lloret and M. Julve, Inorg. Chim. Acta, 2000, 298, 202 CrossRef; (c) C. Ruiz-Pérez, M. Hernández-Molina, J. Sanchiz, T. López, F. Lloret and M. Julve, Inorg. Chim. Acta, 2000, 298, 245 CrossRef.
- B. I. Tsyuk, L. G. Korenera and V. F. Zolen, Koord. Khim., 1977, 3, 465 Search PubMed.
- J. Sun, C. Chen and Z. Qin, J. Lumin., 1988, 40, 246 CrossRef.
- H. K. Henisch, Crystal Growth in Gels, The Pennsylvania State Univ. Press, Pittsburgh, 1970. Search PubMed.
- Enraf-Nonius, CAD-4 EXPRESS, v. 5.1/5.2, Enraf-Nonius, Delft, The Netherlands, 1997..
- A. L. Speck, HELENA: Program for Data Reduction, Utrecht University, The Netherlands, 1997. Search PubMed.
- J. González-Platas and C. Ruiz-Pérez, NEWCORR: Program for Empirical Correction; University of La Laguna, La Laguna, Spain, 1997. Search PubMed.
- A. Altomare, M. C. Burla, M. Camalli, G. Cascarano, C. Giacovazzo, A. Guargliardi, A. G. G. Moliterni, G. , Polidon and R. Spagna, J. Appl. Crystallogr.,in press. Search PubMed.
- G. M. Sheldrick, SHELXL97. Program for the Refinement of Crystal Structure, Universiity of Göttingen, Germany, 1997. Search PubMed.
- M. Nardelli, PARST95 (An update to PARST) a system of Fortran routines for calculating molecular structure parameters from the results of crystal structure analyses. J. Appl. Cryst., 1995, 28, 659. Search PubMed.
- A. L. Spek, Acta Cristallogr. Sect. A, 1990, 46, C-34 Search PubMed.
- E. Hansson, Acta Chem. Scand., 1973, 27, 2827 CrossRef CAS.
- T. Lis, J. Matuszewski and B. Jezowska-Trzebiatowska, Acta Crystallogr. Sect. B, 1977, 33, 1943 CrossRef.
- T. Lis and J. Matuszewski, Acta Crystallogr. Sect. B, 1979, 35, 2212 CrossRef.
- T. Lis and J. Matuszewski, J. Chem. Soc., Dalton Trans., 1980, 996 RSC.
- E. Hansson, Acta Chem. Scand., 1973, 27, 2441 CrossRef CAS.
- E. Hansson, Acta Chem. Scand., 1973, 27, 2813 CrossRef CAS.
- X. Wen Mei, W. Qiguang, Y. Lan and Y. Rudong, Polyhedron, 1992, 11, 2051 CrossRef.
- I. Gil de Muró, F. A. Mautner, M. Insausti, L. Lezama, M. I. Arriortua and T. T. Rojo, Inorg. Chem., 1998, 3243 CrossRef.
- J. G. Kang, S. K. Yoon, Y. Sohn, Y. S. J. G. Kim, Y. D. Kim and I. H. Suh. , J. Chem. Soc., Dalton Trans., 1999, 1467 RSC.
- U. Casellato, R. Graziani, R. P. Bonomo and A. J. D. Bilio, J. Chem. Soc., Dalton Trans., 1991, 23 RSC.
- C. Bellito, F. Federichi and S. A. Ibrahim, Chem. Mater., 1998, 10, 1076 CrossRef.
- A. Cabeza, M. A. G. Aranda and S. Bruque, J. Mater. Chem., 1999, 9, 571 RSC.
| This journal is © The Royal Society of Chemistry 2000 |
