New highly active chiral phosphapalladacycle catalysts. First isolation and characterization of a Pd(IV) intermediate†
Jean Michel Brunel, Marie-Hélène Hirlemann, Andreas Heumann and Gérard Buono*
UMR CNRS 6516, Faculté de St Jérôme, ENSSPICAM, Av. Escadrille Normandie Niemen, 13397, Marseille, Cedex 20, France.. E-mail: buono@spi-chim.u-3mrs.fr
First published on UnassignedUnassigned18th September 2000
Abstract
The synthesis and characterization of a new active P*-active phosphapalladacycle and its successful use in the asymmetric hydroarylation of norbornene with turnover numbers (TONs) of up to 1010 is described.
C–C coupling reactions using numerous transition metals are of fundamental interest in modern synthetic chemistry.1 Thus, the arylation and vinylation of olefins catalyzed by Pd complexes (Heck reaction) has been extensively studied to achieve industrial scale technical application during the last decade.2 In this area, Herrmann et al. discovered in 1995 highly efficient palladacycle catalysts in Heck and related reactions of aryl halides with catalyst turnover numbers (TONs)3 up to 500000.4,5 On the other hand, because of the excellent control of regio- and stereo-selectivity in Heck reactions, intra- and inter-molecular asymmetric variations were developed.6 Nevertheless, all of the results reported in the literature suffer from insufficient catalyst turnover frequencies (TOF<10 h−1 and TON<100) and are limited in the use of aryl triflates and aryl iodides as arylating agents.7 Thus, few syntheses of chiral palladacycle catalysts have been envisioned and all these attempts failed.8 To our knowledge, only Dunina et al. have successfully reported the synthesis of an optically active P*-chiral phosphapalladacycle via resolution of diastereomeric (S)-prolinate derivatives of the racemic dimer.9 In the context of our studies, we have recently described the potentialities of oxaza- and diazaphospholidine ligands in various catalytic enantioselective reactions10 as well as the synthesis of a new class of highly efficient new phosphapalladacycle catalysts for hydroarylation of norbornene with TON levels > 1010.14 Here, we report the synthesis and use of a new chiral phosphapalladacycle catalyst, as well as the isolation of an intermediate palladium complex involved in the hydroarylation of norbornene.
 | ||
| Fig. 1 X-Ray structure of 5, with labeling scheme (for clarity the iodine ion has been omitted). Selected bond distances (Å) and angles (°): P1–Pd2 2.321(3), Pd2–O22 2.1810(1), Pd2–C27 1.221(2), Pd2–C26 2.195(3), C23–O22 1.212(4), P1–N3 1.662(4), P1–N4 1.662(3), P1–C6 1.790(2); Pd2–C27–O22, 115.2(1), P1–Pd2–N3 140.8(1), P1–Pd2–N4 105.7(2), P1–N3–N4 94.5(2), P1–N4–C5 122.1(2), P1–N4–C11 111.4(3), P1–N4–C5 122.1(3), Pd2–C26–C7 132.2(1), Pd2–C26–O22 84.0(1), P1–N3–C14 117.4(4), P1–N3–N4 94.5(3), Pd2–P1–C27 138.4(2), Pd2–P1–C26 86.6(1). | ||
The synthesis of the chiral o-tolyldiazaphospholidine ligand 3 was readily achieved in 89% yield by an exchange reaction in refluxing toluene from bis(dimethylamino)(o-tolyl)phosphine 1 and (S)-anilinomethylpyrrolidine 2 (readily obtained from L-glutamic acid) [eqn. (1)].15 The structure of this ligand was
 | (1) |
Treatment of palladium(II) acetate with ligand 3 in refluxing toluene afforded the expected phosphapalladacycle complex 4 in 75% yield as a thermally, air and moisture stable yellow solid [eqn. (2)]. The structure of this cyclometallated compound,
 | (2) |
 | (3) |
| Entry | Ar–X | Catalyst 4 (mol% Pd) | Conv. (%)a | Yield (%)b | TON | Ee (%)c |
|---|---|---|---|---|---|---|
| a Conversion determined by GC.b Isolated yield.c Enantiomeric excess determined by GC on a fused silica gel column (25 m × 0.25 mm) coated with 10% heptakis(2,3,6-tri-O-methyl)-β-cyclodextrin at 100 °C, tR(−) 39.30 min; tR (+) 40.58 min. | ||||||
| 1 | Ph–I | 0.5 | 100 | 99 | 1.9 × 102 | 14 |
| 2 | Ph–I | 5 × 10−6 | 100 | 98 | 1.9 × 107 | 4 |
| 3 | Ph–I | 5 × 10−7 | 100 | 98 | 1.96 × 108 | 2 |
| 4 | Ph–I | 5 × 10−9 | 100 | 84 | 1.68 × 1010 | 2 |
| 5 | Ph–OTf | 0.5 | 100 | 99 | 1.9 × 102 | 25 |
| 6 | Ph–OTf | 5 × 10−4 | 100 | 99 | 1.98 ×105 | 14 |
As already observed using achiral catalysts, an extraordinary high activity of complex 4 was observed at 120 °C in DMSO.14 Consequently, exceptional turnover numbers up to 1.96 × 108 mol product (mol Pd)−1 and yields of 98% were achieved (Table 1, entries 1–4). In addition, turnover frequencies up to 1.6 × 107 mol product (mol Pd h)−1 were encountered. To our knowledge, these values are the highest TOF reported to date for palladium catalysis involving chiral palladacycle complexes. On the other hand, whatever the experimental conditions, low enantiomeric excesses (ee) up to 25% using phenyl triflate as arylating agent were observed (Table 1, entry 5). Nevertheless, these results constitute the first example of the use of an active chiral P*-phosphapalladacycle catalyst in an enantioselective hydroarylation reaction.
In the course of our investigations on the mechanism (Scheme 1) of this reaction,19 we were able to isolate an intermediate Pd(IV) complex 5 which was fully characterized by X-ray structure analysis (Fig. 1).16,20 Isolation of this intermediate suggests a mechanism for the Heck reaction involving PdII–PdIV species and suggests that the first step of the catalytic cycle is oxidative addition of a haloarene to a palladium(II) compound to form a palladium(IV) species. In the next step, an alkene molecule is coordinated and inserts into the Pd–Ar bond. The insertion process occurs via a four-centered transition state which requires a planar assembly of the alkene and Pd–Ar bond. Hence, insertion proceeds in a syn manner to generate a σ-alkylpalladium complex. The formate anion reacts subsequently with the intermediate 7 producing the norbornyl palladium formate 8 from which exo-phenylnorbornane 9 is generated through decarboxylation and Pd(II) deinsertion.
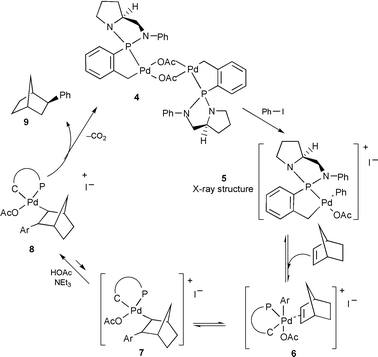 | ||
| Scheme 1 | ||
In conclusion, we have described the synthesis of a new active P*-chiral phosphapalladacycle and its successful use in the asymmetric hydroarylation of norbornene, with TON up to 1010. On the other hand, although the enantiomeric excesses are low (up to 25% ee) they constitute, to our knowledge, the first results in asymmetric catalysis using a chiral phosphapalladacycle catalyst. Further studies relating to structural modification of the chiral ligand are now underway and will be reported in due course.
Notes and references
- A. de Meijere and F. E. Meyer, Angew. Chem., 1994, 106, 2473 CrossRef CAS; A. de Meijere and F. E. Meyer, Angew. Chem., Int. Ed. Engl., 1994, 33, 2379 CrossRef; W. Cabri and I. Candiani, Acc. Chem. Res., 1995, 28, 2 CrossRef; K. C. Nicolaou and E. J. Sorensen, Classics in Total Synthesis, VCH, Weinheim, 1996; Search PubMed; G. Poli, G. Giambastiani and A. Heumann, Tetrahedron, 2000, 56, 5959 Search PubMed.
- W. A. Herrmann, Catalytic Carbon–Carbon Coupling by Palladium Complexes : Heck Reactions in Applied Homogeneous Catalysis with Metal Complexes, eds. B. Cornils, VCH, Weinheim, 1996; Search PubMed; V. V. Grushin and H. Alper, Chem. Rev., 1994, 94, 1047 Search PubMed; R. F. Heck, Palladium Reagents in Organic Synthesis, Academic Press, London, 1985; CrossRef CAS; J. Tsuji, Palladium Reagents, Innovation in Organic Synthesis, Wiley, Chichester, 1995; CrossRef CAS; M. Beller and C. Bolm, Transition Metals for Organic Synthesis, VCH, Weinheim, 1998. CrossRef CAS.
- TON: turnover number [mol product (mol Pd)−1]; TOF: turnover frequency [mol product (mol Pd h)−1]..
- W. A. Herrmann, C. Brossmer, C. P. Reisinger, T. H. Riermeier, K. Ofele and M. Beller, Chem. Eur. J., 1997, 3, 1357 CrossRef CAS; M. Beller, H. Fisher, W. A. Herrmann, K. Öfele and C. Brossmer, Angew. Chem., 1995, 107, 1992 CrossRef.
- It is noteworthy that Milstein and coworkers have also reported the successful use of phosphapalladacycles as catalysts for the Heck reaction: Y. Ben-David, M. Portnoy, M. Gozin and D. Milstein, Organometallics, 1992, 11, 1995 CrossRef CAS.
- M. Shibaski, C. D. J. Boden and A. Kojima, Tetrahedron, 1997, 53, 7371 CrossRef CAS; O. Loiseleur, M. Hayashi, N. Schmees and A. Pfaltz, Synthesis, 1997, 1338 CrossRef CAS; L. E. Overman and D. J. Poon, Angew. Chem., Int. Ed., 1997, 36, 518 CrossRef CAS.
- It is noteworthy that for aryl iodides a stoichiometric amount of silver salts is necessary to obtain good yields and high enantiomeric excesses. See: T. Sakamoto, Y. Kond and H. Yamanaka, Tetrahedron Lett., 1992, 33, 6845 CrossRef CAS; C. Larock, W. H. Gong and B. E. Baker, Tetrahedron Lett., 1989, 30, 2603 CrossRef; T. Sato, S. Nukui, M. Sodeoka and M. Shibasaki, Tetrahedron, 1994, 50, 371 CrossRef.
- M. Beller, T. H. Riermeier, S. Haber, H. J. Kleiner and W. A. Herrmann, Chem. Ber., 1996, 129, 1259 CAS.
- V. V. Dunina, O. N. Gorunova, L. G. Kuz’mina, M. V. Livantsov and Y. K. Grishin, Tetrahedron Asymmetry, 1999, 10, 3951 CrossRef CAS.
- In recent years, we have studied and developed the potentialities of oxazaphospholidine and diazaphospholidine ligands in various catalytic reactions. Some of these systems in which the phosphorus atom is substituted concommitantly with 2 nitrogen and one oxygen atoms or one aromatic carbon atom proved to be excellent mediators for, inter alia, highly selective allylic substitution,11 copper-catalyzed Diels–Alder12 or asymmetric cyclopropanation reactions.13.
- T. Constantieux, J. M. Brunel, A. Labande and G. Buono, Synlett, 1998, 49 CAS; G. Muchow, J. M. Brunel, M. Maffei, O. Pardigon and G. Buono, Tetrahedron, 1998, 54, 10435 CrossRef CAS; J. M. Brunel, T. Constantieux and G. Buono, J. Org. Chem., 1999, 64, 8940 CrossRef CAS.
- J. M. Brunel, B. Del Campo and G. Buono, Tetrahedron Lett., 1998, 39, 9663 CrossRef CAS.
- J. M. Brunel, O. Legrand, S. Reymond and G. Buono, J. Am. Chem. Soc., 1999, 121, 5807 CrossRef CAS.
- J. M. Brunel, A. Heumann and G. Buono, Angew. Chem., Int. Ed., 2000, 39, 1946 CrossRef CAS.
- Ligand 3 was purified by distillation and was obtained as a white air and moisture stable solid in 89% yield on cooling; bp 130 °C (10 Pa); mp 138 °C..
- Crystal data: for 3: colourless cubic monocrystal, C18H20N2P (M = 295.35) obtained by recrystallization from ethyl acetate, approximate dimensions 0.2 × 0.2 × 0.2 mm, monoclinic, space group P21, a = 8.2642(5), b = 8.0347(4), c = 12.4008(8) Å, Z = 2, Dc = 1.22 g cm−3, T = 248 K, Cu-Kα radiation, R = 0.045. For 5: yellow plate monocrystal, C26H28IN2O2PPd (M = 664.81) obtained by recrystallization from ethyl acetate, approximate dimensions 0.3 × 0.4 × 0.5 mm, orthorhombic, space group P2,2,2, a = 9.1514(2), b = 10.8405(4), c = 15.8227(6) Å, Z = 4 , Dc = 1.25 g cm−3, T = 298 K, Cu-Kα radiation, R = 0.044. CCDC 182/1744. See http://www.rsc.org/suppdata/cc/b0/b005521m/ for crystallographic files in .cif format..
- No other isomer was formed, nor was disubstitution observed..
- General information, for the hydroarylation of norbornene see ESI†..
- For some references on mechanistic aspects, see: B. L. Shaw, S. D. Perera and E. A. Staley, Chem. Commun., 1998, 1361 RSC; B. L. Shaw and S. D. Perera, Chem. Commun., 1998, 1863 RSC.
- It is noteworthy that the P, C and O atoms around the Pd center adopt a distorted tetrahedral arrangement with bond angles between 84.0 and 138.4°. The complexation of Pd occurs with retention of configuration at the P atom. The shortness of the P–N3 bond (1.662 cf. 1.701 Å in the free phosphine 3) and of the P–N4 bond (1.662 cf. 1.73 Å) may be assigned to negative hyperconjugation due to electron donation from πN to σ*P–Pd orbitals. See: D. G. Gilheany, Chem. Rev., 1994, 94, 1339..
Footnote |
| † Electronic supplementary information (ESI) available: Fig. SI 1 (structure of 3, with labelling scheme), physical and spectroscopic data for 3, and general information for norbornene hydroarylation. See http://www.rsc.org/suppdata/cc/b0/b005521m/ |
| This journal is © The Royal Society of Chemistry 2000 |
