Luminescent molecular logic gates: the two-input inhibit (INH) function
Thorfinnur Gunnlaugsson*a, Dónall A. Mac Dónaila and David Parkerb
aDepartment of Chemistry, University of Dublin, Trinity College Dublin, Dublin 2, Ireland.. E-mail: gunnlaut@tcd.ie
bDepartment of Chemistry, University of Durham, South Road, Durham, UK DH1 3LE
First published on UnassignedUnassigned7th January 2000
Abstract
The Tb(III) complex 3, is the first example of a molecular logic gate corresponding to a two-input INHIBIT function, A ⁁ B′ where the ‘output’, a sharp, line-like, terbium emission, is only observed with two chemical inputs (i) the presence of protons and (ii) the absence of molecular oxygen.
Developments in supramolecular chemistry and nanotechnology have stimulated interest in the construction of simple electronic or photonic driven systems and networks that function as molecular-level devices.1 Examples include simple host–guest complexes as well as more advanced switches,2 wires,3 grids, 4 shuttles5 and molecular machines.6 Mimicking the functions of semiconductor logic gates used in modern computing is of particular interest,7 where the relationship between input and the output may be described by truth tables, where 1 represents an active input/output and 0 an inactive one.8 For a two input system there are 16 different logic gate functions.8b† Some of these functions have recently been demonstrated where ions and molecules are used as inputs,7 including the ID (or YES), AND, OR and NOT logic functions designed by de Silva et. al.9 and the XOR function designed by Balzani et al.10 Among the remaining logic gates is the inhibit (INH) function which can be interpreted as a particular integration of an AND and a NOT logic functions, where the output signal is inhibited by one of the active inputs.‡ Recently the more complex three-input integrated INH logic gate was demonstrated.11 However, the fundamental two-input INH gate has not been reported.
Here, we demonstrate that in water the Tb(III) based quinolyl derived macrocyclic 1,4,7,10-tetraazacyclododecane (cyclen) conjugate, 3, yields such an INH logic gate. The two inputs are H+ (the nitrogen moiety of the quinoline acting as a proton acceptor) and O2 (or rather the absence of O2, since this input is not asserted when O2 is present). The output signal is a delayed line like Tb luminescence (owing to the deactivation of the Tb 5D4 excited state to the 7FJ, J = 6, 5, 4 and 3), occurring at long wavelengths. These are important features since sharp, long wavelength emissions give minimal signal interference, critical for high performance signalling systems. Tb was chosen because, unlike Eu(III), pyridine based Tb and related complexes are known to be spectroscopically sensitive to O2,12 opening the possibility of a gate with O2 as a second input. Moreover, lanthanide based cyclen derivatives have been shown to be kinetically stable in water.13
The syntheses of the α-chloroamide 4, the free ligand in 1 and the Eu complex 2, have previously been described; 2 was designed as a delayed Eu luminescence pH sensor.143, the Tb complex of 1, was synthesised in a similar manner to 2, and obtained in 55% yield after treating 1 with Tb(CF3SO3)3 in refluxing acetonitrile, followed by a purification on alumina. Complexation was established by electrospray (ES+) mass spectrometry and 31P NMR.§
The absorption spectrum of 3 showed a similar pH dependence to 2 and 4, with λmax at 314 nm (log ε = 4.18) and a band at 261 nm under acid conditions with λmax shifting to 295 nm (log ε = 4.0) in the presence of base (isosbestic points at 299 and 271 nm). The Eu luminescence (occurring from 5D0, E = 17200 cm−1)15 of 2 (1 × 10−5 mol dm−3) in H2O under ambient conditions ([O2] ca. 0.23 mmol dm−3) was highly pH dependent when excited at 330 nm, with a 250 fold luminescence enhancement (LE) observed upon addition of acid ([CF3CO2H] = 3 mmol dm−3/pH = 2.5). This pH dependence signals the protonation of the remote quinoline nitrogen moiety (pKa = 5.8) and the ‘tuning’ of the so-called antenna effect, the energy transfer mechanism from the singlet excited state (S1) of the chromophore (via the triplet, T1) to the lanthanide excited state.12,13¶
The Tb luminescence of 3 (appearing at 491.5, 547.5, 588.0 and 623.0 nm, J = 6, 5, 4 and 3 respectively) was somewhat different. The excited state emission from 5D4 (E = 20500 cm−1) was only weakly observed in alkaline solution under ambient conditions, with only a 1.7 fold enhancement upon acidification as seen in Fig. 1(a) ([CF3CO2H] = 1.26 mmol dm−3/pH = 2.9) when excited at 330 nm. The corresponding fluorescence changes were more apparent; both a hypsochromic shift from 373 to 357 nm (isoemissive point at 387 nm) and intensity changes were observed upon acidification (two shoulders at 371 and 345 nm).
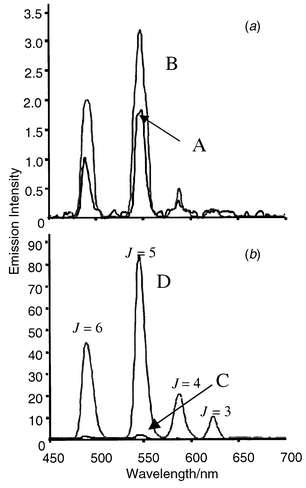 | ||
| Fig. 1 Tb(III) emission of 3 (1 × 10−5 mol dm−3) in water at 293 K, 10.0 mmol dm−3 NMe4ClO4 (to maintain constant ionic strength): (a) in aerated solution at: (A) pH = 11, (B) pH = 2.9; (b) in degassed solution at: (C) pH = 11, (D) pH = 2.9. Note the difference in the intensity scales. | ||
The measurements were repeated in degassed solution (‘freeze–pump–thaw’, [O2] < 10−6 mol dm−3). In alkaline solution the Tb emission spectrum and lifetime (τTb = 0.39 ms) were unaffected (owing to the inefficient population of the lanthanide excited state¶). However, in acid solution degassing yielded a ca.50 fold increase in the Tb luminescence intensity at 547.5 nm [Fig. 1(b)], and an almost threefold increase in the lifetime (τTb = 0.98 ms); these conditions ‘switch on’ the Tb emission [note the different scales in Fig. 1(a) and 1(b)]. The fluorescence spectra were however only marginally affected by the removal of O2.
The small Tb emission in aerated acidic solution arises from the fact that the T1 state of the quinoline (T1E = 21978 cm−1, as measured for the protonated form of 4) is partly quenched by an ambient level of O2. The Tb 5D4 state can also participate in a back-energy transfer mechanism to the T1 state (the energy difference being 1478 cm−1); these combined processes lead to efficient quenching of the 5D4 state (i.e. the Tb emission is switched off). Upon removal of O2 this quenching process is largely suppressed leading to more efficient population of the Tb 5D4 state (the antenna effect is more efficient) and switching on of the Tb emission.12
From the perspective of logic functions, the Eu emission (2)is determined only by the presence of single ionic input (H+), back energy transfer from 5D0 is inefficient and the complex is thus insensitive to O2,7 and can as such be regarded as a pass logic function (YES9 or ID8b logic gate). The emission of the Tb complex (3) is more interesting and is switched on only in the presence of H+ and absence of O2. Under other circumstances, the absence of H+, the presence of O2, or both, no significant Tb emission is observed (based on discrimination in LE factors; ca. 50 when O2absent cf. 1.7 with O2present, at 547.5 nm, Fig. 1). This behaviour may be conveniently described using logic notation,7,9 written A ⁁ B′, where A and B represent the H+ and O2 inputs respectively. The corresponding truth table is shown in Fig. 2(a), where an active output (X = 1, corresponding to emission) is obtained only when A = 1 and B = 0.8 This logic gate is the inhibit (INH) function, Fig. 2(b).
![(a) A truth table for the INH logic gate; inputs A and B correspond to
the H+ and O2 respectively; X [Tb(iii)
emission], is the output signal. (b) The INH gate represented using a
conventional gate notation; an active output signal (Tb emission) is
obtained when A = 1 and B = 0.](/image/article/2000/CC/a908951i/a908951i-f2.gif) | ||
| Fig. 2 (a) A truth table for the INH logic gate; inputs A and B correspond to the H+ and O2 respectively; X [Tb(III) emission], is the output signal. (b) The INH gate represented using a conventional gate notation; an active output signal (Tb emission) is obtained when A = 1 and B = 0. | ||
Even though more advanced practical integration of such molecular level logic gates into circuits is somewhat beyond the horizon, progress depends of the generation of all fundamental logic operations. Our goal was to generate a two-input INH logic gate, and the Tb emission based gate reported herein is the first example of such a two-input molecular gate. Importantly, unlike many previous molecular logic gates where the output is a broad emission,7,9,11 the output signal for 3, is a set of line-like Tb emission bands occurring at long wavelengths (Stokes shifts of 160–300 nm for J = 6–3, respectively) with narrow bandwidth (ca. 10 nm) giving rise to a high signal quality. Complex 3, is thus an important contribution to the development of molecular logic devices, not least since the current silicon based computer chips are expected to reach their physical limits in the near future.16
Acknowledgements
We are grateful to the BBSRC and Kinerton Ltd. for financial support, Professor John M. Kelly, Dr J. C. Penedo Esteiro and Dr Hazel M. Moncrieff for valuable discussions.References
- Molecular Electronics and Molecular Electronic Devices, ed. K. Sienicki, CRC Press, USA, vol. 1, 1993; J.-M. Lehn, Search PubMed; Supramolecular Chemistry, Concepts and Prespectives, VCH, Weinheim, 1995; Search PubMed; V. Balzani and F. Scandola, Supramolecular Photochemistry, Ellis Horwood, Chichester, 1991. Search PubMed.
- P. R. Ashton, V. Balzani, J. Becher, A. Credi, M. C. T. Fyfe, G. Mattersteig, S. Menzer, M. B. Nielsen, F. M. Raymo, J. F. Stoddart, M. Venturi and D. J. Williams, J. Am. Chem. Soc., 1999, 121, 3951 CrossRef CAS; A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, A. J. M. Huxley, C. P. McCoy, J. T. Rademacher and T. E. Rice, Chem. Rev., 1997, 97, 1515 CrossRef.
- R. Ziessel and A. Harriman, Coord. Chem. Rev., 1998, 171, 331 CrossRef; A. Harriman and R. Ziessel, Chem. Commun., 1996, 1707 RSC.
- A. M. Garcia, D. M. Bassani, J.-M. Lehn, G. Baum and D. Fenske, Chem. Eur. J., 1999, 5, 1234 CrossRef CAS; L. DeCola, Chimia, 1996, 50, 214 Search PubMed.
- D. J. Cardenas, D. J. Collin, P. Gavina, J. P. Sauvage, A. De Cian, J. Fischer, N. Armaroli, L. Flamigini, V. Vicinelli and V. Balzani, J. Am. Chem. Soc., 1999, 121, 5481 CrossRef CAS; R. A. Bissel, E. Cordova, A. E. Kaifer and J. F. Stoddart, Nature, 1994, 369, 133 CrossRef.
- N. Koumura, R. W. J. Zijlstra, R. A. van Delden, N. Harada and B. L. Feringa, Nature, 1999, 401, 152 CrossRef CAS; C. D. Mao, W. Q. Sun, Z. Y. Shen and N. C. Seeman, Nature, 1999, 397, 144 CrossRef CAS; V. Balzani, M. GomezLopez and J. F. Stoddart, Acc. Chem. Res., 1998, 31, 405 CrossRef CAS.
- A. P. de Silva, H. Q. N. Gunaratne and C. P. McCoy, Nature, 1993, 364, 42 CrossRef; C. P. Collier, E. W. Wong, M. Belohradsky, F. M. Raymo, J. F. Stoddart, P. J. Kuekes, R. S. Williams and J. R. Heath, Science, 1999, 285, 391 CrossRef CAS; M. Asakawa, P. R. Ashton, V. Balzani, A. Credi, G. Mattersteig, O. A. Matthews, M. Montalti, N. Spencer, J. F. Stoddart and M. Venturi, Chem. Eur. J., 1997, 7, 1992; C. R. Copper and T. D. James, Chem. Commun., 1997, 1419 RSC; P. Ghosh, P. K. Bharadwaj, S. Mandal and S. Ghosh, J. Am. Chem. Soc., 1996, 118, 1553 CrossRef CAS; S. Iwata and K. Tanaka, J. Chem. Soc., Chem. Commun., 1995, 1491 RSC.
- (a) R. H. Katz, Contemporary Logic Design,Benjamin Cummings, CA 1994; Search PubMed; (b) P. Burger, Digital Design: A Practical Course,Wiley, New York, 1988. Search PubMed.
- A. P. de Silva, H. Q. N. Gunaratne and C. P. McCoy, J. Am. Chem. Soc., 1997, 119, 7891 CrossRef CAS; A. P. de Silva, H. Q. N. Gunaratne, T. Gunnlaugsson, C. P. McCoy, P. R. S. Maxwell, J. T. Rademacher and T. E. Rice, Pure Appl. Chem., 1996, 68, 1443 CrossRef CAS; A. P. de Silva, H. Q. N. Gunaratne and G. E. Maguire, J. Chem. Soc., Chem. Commun., 1994, 1213 RSC.
- V. Balzani, A. Credi and M. Venturi, Coord. Chem. Rev., 1998, 171, 3 CrossRef CAS; A. Credi, V. Balzani, S. J. Langford and J. F. Stoddart, J. Am. Chem. Soc., 1997, 119, 2679 CrossRef CAS.
- A. P. de Silva, I. M. Dixon, H. Q. N. Gunaratne, T. Gunnlaugsson, P. R. S. Maxwell and T. E. Rice, J. Am. Chem. Soc., 1999, 121, 1393 CrossRef CAS.
- N. Sabbatini, M. Guardigli and J.-M. Lehn, Coord. Chem. Rev., 1993, 123, 201 CrossRef CAS; D. Parker and J. A. G. Williams, Chem. Commun., 1998, 245 RSC; A. P. de Silva, H. Q. N. Gunaratne, T. E. Rice and S. Stewards, Chem. Commun., 1997, 1891 RSC; J.-C. G. Bunzli, in Lanthanide Probes in Life, Chemical and Earth Sciences, Theory and Practice, ed. J.-C. G. Bunzli and G. R. Choppin, Elsevier, New York, 1989, p. 219. Search PubMed.
- D. Parker and J. A. G. Williams, J. Chem. Soc., Dalton Trans., 1996, 3613 RSC; D. Parker and J. A. G. Williams, J. Chem. Soc. Perkin Trans., 2, 1996, 1581 RSC.
- T. Gunnlaugsson and D. Parker, Chem. Commun., 1998, 511 RSC.
- G. Stein and E. Würzberg, J. Chem, Phys., 1975, 62, 208 CrossRef CAS.
- D. A. Muller, T. Sorsch, S. Moccio, F. H. Baumann, K. Evans-Lutterodt and G. Timp, Nature, 1999, 399, 759 CrossRef CAS.
Footnotes |
| † The ONE and ZERO functions are trivial, the output being a 1 or 0 respectively, regardless of the input. Four functions depend on a single input, namely both ID and both INV (or NOT) functions. The remaining ten are functions of two inputs, and of these AND, OR, XOR, NAND, NOR and EQU (or XNOR) satisfy commutation. The last four functions corresponding to INH and IMP gates do not satisfy commutation. |
| ‡ The INH function, A ⁁ B′, should not be confused with the NAND function, (A ⁁ B)′. |
| § ES+: m/z 802.94 (15, M+ + 1), 826.30 (13, M + Na+). 31P NMR (101 MHz, CH3CN): δ 441.71 (br s) and 427.76 (br s) cf. δ 42.95 and 33.54 for 1. |
| ¶ Population of the quinoline S1 (λex = 330 nm) and subsequent population of the lanthanide excited state (the antenna effect) is inefficient in alkaline solution since the chromophore is only weakly absorbing at this wavelength. On acidification the S1 population is greatly enhanced since absorption is shifted to longer wavelengths. |
| This journal is © The Royal Society of Chemistry 2000 |

