Total spontaneous resolution of a cyanoguanidine showing only conformational chirality
Ian D.
Cunningham
*a,
Simon J.
Coles
b and
Michael B.
Hursthouse
b
aDepartment of Chemistry, School of Physics and Chemistry, University of Surrey, Guildford, UK GU2 5XH. E-mail: i.cunningham@surrey.ac.uk
bDepartment of Chemistry, University of Southampton, Highfield, Southampton, UK SO17 1BJ
First published on 6th January 2000
Abstract
The total spontaneous resolution of N-(4-chlorophenyl)-N′-cyano-N,N″ ,N″-trimethylguanidine, a compound which shows only conformational chirality, is described; variable temperature NMR experiments show that rotation of the dimethylamino group is rapid in solution at room temperature as is conformer interconversion by rotation of the arylmethylamino group.
Total spontaneous resolution is a relatively rare phenomenon whereby a racemic mixture crystallises as a single enantiomer.1 It may be seen where a potentially chiral molecule is configurationally labile in solution, but stable in the solid state. These criteria are most commonly met when chirality is due solely to a chiral conformation and to the maintenance of this conformation by topology in the solid state. Despite its relative rarity, examples of total spontaneous resolution are known and the phenomenon is best illustrated by rac-1,1′-binaphthyl which has been known to crystallise in up to 95% ee.2
What raises the phenomenon of total spontaneous resolution above a mere curiosity is its importance in crystal engineering,3 its potential for asymmetric synthesis starting from achiral reagents,4 and its relevance to the origin of enantiomeric homogeneity in nature.5
Of course many organic compounds, while lacking a ‘traditional’ asymmetric carbon, adopt a chiral conformation in the solid state. However, as with more configurationally stable chiral compounds, crystallisation as a racemic compound is most common, or in certain cases as a conglomerate.6 Examples of the latter are not uncommon, and the synthetic use of homochiral crystals has been reviewed.7 However, in many cases the so-called ‘absolute asymmetric synthesis’ is based on selection of a ‘single crystal’ from what may well be a conglomerate, or on use of homochiral crystals obtained by seeding.8 What is of more fundamental interest is where such a chiral conformer can not only be isolated in the crystalline state, but can be shown to be an example of total spontaneous resolution. Total spontaneous resolution is usually described for examples such as 1,1′-binaphthyl where racemization following dissolution is slow enough to be detected (kracca. 10−2 s−1). However, such examples are very rare and if studies can be extended to include solid state conformational isomers where the racemization in solution is fast (kracca. 104 s−1), the scope of these studies can be greatly increased. We herein report such a material.
The compound N-(4-chlorophenyl)-N′-cyanoguanidine 1, prepared from 4-chloroaniline and dicyanamide,9 is weakly acidic.10 Treatment of 1 with BuLi and excess MeI yielded N-(4-chlorophenyl)-N′-cyano-N,N″,N ″-trimethylguanidine 2 (Scheme 1) as a mass of small microcystals (56 mg, 17% yield).11 Preliminary X-ray crystallographic analysis of one of these crystals indicated a chiral space group P212121. Since no chirality was present in the starting material or in any of the reagents used to prepare 2, this suggested a chiral conformation in the solid state. At this stage it was not possible to distinguish whether this material existed as a conglomerate or was an example of total spontaneous resolution. Therefore, a sample comprising 18.2 mg was selected at random from the fine mass, dissolved in CH2Cl2 and allowed to recrystallise by slow evaporation of solvent to yield a single crystal of weight 16.2 mg (89% recovery).
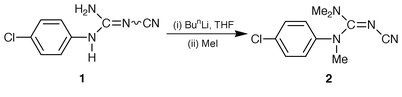 | ||
| Scheme 1 | ||
X-Ray crystallographic analysis of a carefully cut piece of this single crystal again showed the P212121 space group and the chiral conformation shown in Fig. 1 and schematically in Fig. 2;12 the absolute configuration was aS.13
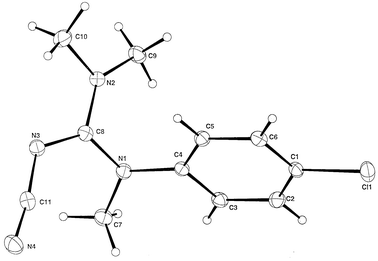 | ||
| Fig. 1 ORTEP diagram of compound 2; The numbering is arbitrary. | ||
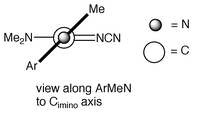 | ||
| Fig. 2 Schematic representation of the conformation of 2. | ||
The chirality arises due to the twisting of the ArMeN portion relative
to the rest of the molecule by ca. 47°
(C4–N1–C8–N2). The twisting of the ArMeN group is all the
more remarkable in that it results in loss of conjugation between the ArMeN
lone pair and the C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N system. This is reflected in the increased
length of 1.385(2) Å for the ArMeN–C bond compared to the
Me2N–C and C
N system. This is reflected in the increased
length of 1.385(2) Å for the ArMeN–C bond compared to the
Me2N–C and C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N bonds at 1.340(2) and 1.325(2)
Å, respectively.14 The lack of
planarity can be attributed to ‘steric crowding’, and the fact
that the ArMeN nitrogen lone pair can delocalise ‘towards’ the
Ar, as an alternative to the C
N bonds at 1.340(2) and 1.325(2)
Å, respectively.14 The lack of
planarity can be attributed to ‘steric crowding’, and the fact
that the ArMeN nitrogen lone pair can delocalise ‘towards’ the
Ar, as an alternative to the C![[double bond, length half m-dash]](https://www.rsc.org/images/entities/char_e006.gif) N–CN, probably explains why
this group, rather than the Me2N, is rotated.
N–CN, probably explains why
this group, rather than the Me2N, is rotated.
The following facts prove that this is an example of total spontaneous resolution of a chiral conformer. Firstly, the X-ray crystallographic analysis shows that the whole crystal consists of only one chiral conformer [Flack x parameter = 0.02(6)15]. Secondly, this single crystal constitutes an almost quantitative yield of precipitate from homogeneous solution. Thirdly, the homogeneous solution was obtained using a relatively large randomly selected sample of the original material.16
Interestingly, in acetone-d6 solution the formally non-equivalent dimethyl groups (of Me2N) separated into two peaks at low temperature and variable temperature NMR experiments gave a coalescence temperature of 231 K. A value for ΔG‡rot of 46 ± 1 kJ mol−1 is obtained from eqn. (1) where R, h and kb are gas, Planck and Boltzman constants, respectively, and Tc is the coalescence temperature).17
| ΔG‡rot = −RTcln[πh(Δν)/1.41 42kbTc](1) | (1) |
Experimental support for possible ‘coupled’ rotation of the Me2N and ArMeN groups comes from the NMR. On warming from belowTC, one of the methyl peaks (that at higher δ) becomes noticeably broader than the other one (see Fig. 3: NMR spectrum at 223 K, Me2N signals at δ 3.10 and 2.85) before a broad but symmetrical peak is obtained on coalescence. We attribute this to restricted rotation of the methyl closer to the ArMeN group because of mutual steric interaction as the Me2N fragment begins to rotate ‘past’ the ArMeN; some evidence of broadening of the aryl ortho hydrogens is also evident in the NMR spectrum at 223 K.
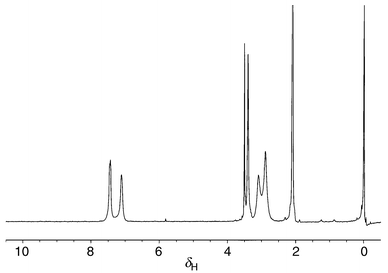 | ||
| Fig. 3 Spectrum of 2 in acetone-d6 at 223 K. Peaks at ca. δ 2.0, 3.4 and 3.5 are due to residual acetone, the ArMeN and water, respectively. | ||
We are grateful to Dr D. C. Povey and Mr G. W. Smith of the University of Surrey for some preliminary X-ray crystallographic studies.
References
- E. L. Eliel and S. H. Wilen, Stereochemistry of Organic Compounds, Wiley, New York, 1994, ch. 7. Search PubMed.
- K. R. Wilson and R. E. Pincock, J. Am. Chem. Soc., 1975, 97, 1474 CrossRef CAS; R. Kuroda and S. F. Mason, J. Chem., Soc., Perkin Trans. 2, 1981, 167 RSC.
- G. R. Desiraju, Angew. Chem., Int. Ed. Engl., 1995, 34, 2311; Search PubMed; G. R. Desiraju, Crystal Engineering. The Design of Organic Solids, Materials Science Mongraphs 54, Elsevier, Amsterdam, 1989. Search PubMed.
- Defined as absolute asymmetric synthesis, see M. Sakamoto, Chem. Eur. J., 1997, 3, 684 and references therein. Search PubMed.
- L. Addadi and M. Lahav, Origins of Optical Activity in Nature, ed. D. C. Walker, Elsevier, Amsterdam, 1979, ch. 14; Search PubMed; E. L. Eliel and S. H. Wilen, Stereochemistry of Organic Compounds, Wiley, New York, 1994, ch. 6. Search PubMed.
- A racemic compound is where a particular chiral molecule is partnered within the crystal by its enantiomer. A conglomerate is a mixture of crystals, each of one or the other chiral form; J. Jacques, A. Collet and S. H. Wilen, Enantiomers, Racemates and Resolutions, Wiley, New York, 1981. Search PubMed.
- 7 See ref 4.With a conglomerate where the molecules owe their chirality only to conformational differences, selection of a single homochiral crystal followed by a suitable transformation can yield a configurationally stable chiral product and qualify as an absolute asymmetric synthesis. However, a pedant might point to the requirement of a chiral human auxilliary!.
- See for example, T. Suzuki, T. Fukushima, Y. Yamashita and T. Miyashi, J. Am. Chem. Soc., 1994, 116, 2793; Search PubMed; A. Sekine, K. Hori, Y. Ohashi, M. Yagi, M. Toda and F. Toda, J. Am. Chem. Soc., 1989, 111, 697; Search PubMed; M. Sakamoto, M. Takahashi, T. Fujita, S. Watanabe, I. Iida, T. Nishio and N. H. Aoyama, J. Org. Chem., 1993, 58, 3476. Search PubMed.
- I. D. Cunningham, B. G. Cox and N. C. Wan, J. Chem. Soc., Perkin Trans. 2, 1994, 1849 RSC.
- I. D. Cunningham, B. G. Cox and N. C. Wan, J. Chem. Soc., Perkin Trans. 2, 1999, 693 RSC.
- The remaining ‘yield’ was made up of a monomethylated product, a dimethylated product, recovered starting material and a cyanamide product (See ref. 10)..
- Crystal data for 2: C11H13ClN4, M = 236.7, colourless, 0.35 × 0.15 × 0.15 mm, orthorhombic, P212121, a = 6.0730(10), b = 13.401(3), c = 14.091(3) Å, V = 1146.8(4) Å3, T = 150(2) K, Z = 4, μ = 0.311 mm−1, 7762 reflections collected, 2630 independent reflections, R = 0.0351, Rw = 0.0885. The structure solution was by direct methods with refinement by full-matrix least squares on F2. CCDC 182/1491..
- See E. L. Eliel and S. H. Wilen, Stereochemistry of Organic Compounds, Wiley, New York, 1994, ch. 14, p. 1120 for rules for specifying configuration due to axial chirality. Search PubMed.
- The less substituted and more planar N-cyano-N-(4-methoxyphenyl)guanidine has bond lengths of 1.328, 1.317 and 1.342 Å for the analogous bonds (see I. D. Cunningham, N. C. Wan, D. C. Povey, G. W. Smith and B. G. Cox, Acta Crystallogr., 1997, C53, 984). Search PubMed; See also ‘mean geometry’ for guanidines from CCDC data, T. Krigowski and K. Wozniak, in Chemistry of Amidines and Imidates, Vol. 2, ed. S. Patai and Z. Rappoport, Wiley, Chichester, 1991, p116. Search PubMed.
- H. D. Flack, Acta Crystallogr., 1983, A39, 876 CrossRef CAS; G. Bernardinelli and H. D. Flack, Acta Crystallogr., 1985, A41, 500 CrossRef.
- It is quite possible that the original sample had already undergone total spontaneous resolution, but with a material which is conformationally labile in solution and which consists of a large number of microcrystals proving this is almost impossible..
- Experimental and calculated values for guanidinium range 20–81 kJ mol−1, C. L. Perrin, in Chemistry of Amidines and Imidates, Vol. 2, ed. S. Patai and Z. Rappoport, Wiley, Chichester, 1991, p. 201; Search PubMed; Y. Yamamoto and S. Kojima, in Chemistry of Amidines and Imidates, Vol. 2, ed. S. Patai and Z. Rappoport, Wiley, Chichester, 1991, p. 509. Search PubMed.
- A concerted rotation has been observed for a tertiary aromatic amide where, as here, planarity is lost due to ‘steric crowding’, J. Clayden and J. H. Pink, Angew. Chem., Int. Ed., 1998, 37, 1937. Search PubMed.
| This journal is © The Royal Society of Chemistry 2000 |
