A polymeric, layered bimetallic Mn(II)Fe(III) imidazolate network; crystal structure and magnetic properties
François Lambert*a, Jean-Philippe Renaulta, Clotilde Policara, Irène Morgenstern-Badaraua and Michèle Cesariob
aLaboratoire de Chimie Bioorganique et Bioinorganique, Institut de Chimie Moléculaire d’Orsay, Université Paris-Sud, F-91405, Orsay, France.. E-mail: flambert @icmo.u-psud.fr
bInstitut de Chimie des Substances Naturelles, CNRS, F-91198, Gif-s/-Yvette, France.
First published on UnassignedUnassigned6th January 2000
Abstract
The use of a C3 complex bearing three imidazolate groups as a ‘building block’ allowed us to synthesize an infinite corrugated two-dimensional (2D) low-spin Fe(III)–high-spin Mn(II) polymer exhibiting weak ferromagnetic intralayer interaction through imidazolate bridge.
Imidazolate is a highly studied bridge in dimetallic compounds,1a as models of (Cu–Zn)SOD1b,c and cytochrome c oxidase.1d Imidazolate has been previously used in building two- or three-dimensional homo or poly-metallic polymers2a,b and is known to induce moderate magnetic coupling.3
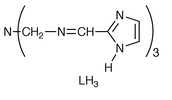
In order to construct bidimensional structures, we used a pseudo-octahedral iron complex from a N-tripodal ligand (LH3 = tris{[2-(2-imidazol-2-yl)methyl]iminoethyl}amine) bearing an imidazolate group at the end of each chain as a building block. A dimer based on an analogous iron complex with imidazole substituted in the 4-position has been previously prepared.2c We chose a 2-substituted imidazole group to design a more planar M1–imidazolate-M0–imidazolate–M1 geometry.† The iron monomer used is a stable neutral triimidazolate Fe(III) low-spin complex.4
Here, we report the X-ray structure of [Mn(hfac)2]3[Fe(L)]2 (hfac = 1,1,1,5,5,5-hexafluoroacetylacetonate), an infinite 2D polymer and a preliminary magnetic study of this compound.
Small dark-blue crystals suitable for X-ray diffraction‡ were obtained readily by slow evaporation of a mixture of methanolic solutions of the iron and manganese complexes [Fe(L)] and [Mn(hfac)2].§ X-Ray crystallography shows an infinite honeycomb-like layered structure in the product. Each Fe(III)(L) entity is linked by imidazolate bridges with three Mn(II), whereas each Mn(hfac)2 is bound in cis position to two Fe(III) complexes. The iron atoms are alternately capped above and below by the tripodal ligand L [Fig. 1(a)]. Within each layer the iron complexes display the same helicity, however, the crystal is non-chiral as revealed by the space group. The iron atoms of a given layer are located at the vertices of a chair-like structure (see Fig. 2) leading to a corrugated 2D-network similar to the Cr–Ni cyanide-bridged compound described by Ferlay et al.5a A remarkable feature is the cis substitution of imidazolate bridges around the manganese. This probably favours overall compactness and therefore the formation of a very stable hexagonal structure.5a,b Also, the small imidazolate–Mn–imidazolate angles) compensate for the large Mn–imidazolate–Fe–imidazolate–Mn distance.
 | ||
| Fig. 1 (a) View of a sheet down the c axis. H atoms and F atoms have been omitted for clarity. Shortest Fe⋯Mn, Fe⋯Fe and Mn⋯Mn separations are 5.889(3), 11.26(1) and 9.62(1) Å, respectively. The Mn–Fe–Mn angle is 109.5°; (b) Molecular structure of two iron units bonded to one manganese unit. H atoms have been omitted for clarity. Each F atom has two partially occupied positions with those shown being in the major sites. Selected bond lengths (Å): Fe–N 3.130(10), Fe–N(1A) 1.924(5), Fe–N(7A) 1.983(5), Mn–N(4A) 2.176(5), Mn–O(1) 2.209(6), Mn–O(2) 2.164(5). Symmetry operations: i = x, y, z; ii = x − y + 1/3, − y + 2/3, −z + 1/6). | ||
 | ||
| Fig. 2 View of the iron–manganese network showing the corrugated structure of a layer. Only two adjacent chair-like arrangements are represented. | ||
The magnetic properties of [Mn(hfac)2]3[Fe(L)]2 (Fig. 3) were recorded using a SQUID magnetometer with an applied field H = 1 kOe in the temperature range 50–300 K and with an applied field H = 100 Oe in the temperature range 2–50 K to avoid saturation.¶ At 300 K χMT = 14.1 cm3 K mol−1, in good agreement with the expected value for two non-coupled low-spin iron(III) (g = 2.28) and three high-spin manganese(II) (g = 2) ions.|| Upon cooling, χMT increases and reaches a value of 45 cm3 K mol−1 at 2 K. This behavior indicates the occurrence of a short range ferromagnetic exchange coupling between low-spin Fe(III) and high-spin Mn(II) through the imidazolato bridge. The variation of magnetization as a function of field has also been measured at 2 K. At saturation, expected values would be 17 μB (S = 17/2) and 13 μB (S = 13/2) for ferromagnetic and antiferromagnetic coupling, respectively (assuming an average g-value of 2). The observed magnetization reaches a value of 16.27 μB per Fe2Mn3 unit at 5.5 kOe which clearly indicates a ferromagnetic coupling. The overall shape of the M = f(H) curve and the low value (45 cm3 K mol−1) observed at 2 K for χMT shows only weak magnetic correlation within a layer,** restricted approximately to one Fe2Mn3 unit. Moreover, no interaction between the layers is observed down to 2 K. The compound can be considered at most as two dimensional from the magnetic point of view.
 | ||
| Fig. 3 Experimental temperature dependence of χMT (χM susceptibility). From 300 to 50 K, the magnetization was recorded with an applied field of 10 kOe, and from 50 to 2 K with an applied field of 100 Oe. The diamagnetic contribution of the ligands has been subtracted as usual. | ||
This compound is a good example of the ability for C3 complexes, derived from a tripodal ligand bearing three bridging groups, to be a building block for 2D polymers. As far as we know, it is the first example of a 2D-bimetallic compound containing imidazolate bridges.6
Acknowledgements
We thank Dr E. Rivière for his contribution to magnetic measurements and Professor T. Mallah for useful discussions.References
- (a) H. M. Hu, H. S. Sun, Q. Zhao, Z. Yu, X.Y. Huang and X. Z. You, J. Coord. Chem., 1998, 43, 361 Search PubMed; (b) G. Tabbi, W. L. Driessen, J. Reedijk, R. P. Bonomo, N. Veldman and A. Spek, Inorg. Chem., 1997, 36, 1168 CrossRef CAS; (c) J. L. Pierre, P. Chautemps, S. Refaif, C. Begin, A. El Marzouki, G. Serratrice, E. Saint-aman and P. Rey, J. Am. Chem. Soc., 1995, 117, 1965 CrossRef CAS; (d) J. T. Landrum, C. A. Reed, K. Hatano and W. R. Scheidt, J. Am. Chem. Soc., 1978, 37, 3232 CrossRef.
- (a) E. Colacio, J. M. Dominguez-vera, M. Ghazi, R. Kivekäs, M. Klinga and J. M. Moreno, Inorg. Chem., 1998, 37, 3040 CrossRef CAS; (b) S. J. Retting, A. Storr, D. A. Summers, R. C. Thompson and J. Troter, J. Am. Chem. Soc., 1997, 119, 8675 CrossRef; (c) C. T. Brewer, G. Brewer, M. Shang, W. R. Scheidt and I. Muller, Inorg. Chim. Acta, 1998, 278, 197 CrossRef CAS.
- G. Kolks, S. J. Lippard, J. V. Waszczak and H. R. Lilienthal, J. Am. Chem. Soc., 1982, 104, 717 CrossRef CAS.
- F. Lambert, J. P. Renault, C. Policar, I. Morgenstern-Badarau, M. Cesario, J. Guilhem, B. Keita and L. Nadjo, manuscript in preparation..
- (a) S. Ferlay, T. Mallah, J. Vaissermann, F. Bartolomé, P. Veillet and M. Verdaguer, Chem. Commun., 1996, 2481 RSC; (b) S. Decurtins, H. W. Schmalle, H. R. Oswald, A. Linden, J. Ensling, P. Gütlich and A. Hauser, Inorg. Chim. Acta, 1994, 216, 65 CrossRef CAS.
- We thank one of the referees for having suggested this..
Footnotes |
| † The Mn–Fe–Mn angle for the present compound is 109°. For a 4-imidazolate iron complex this angle is estimated to be 80°. |
‡ Crystal data:
[C66N20O12H48F36Mn
3Fe2]n, M = 2273.76,
trigonal, space group R![[3 with combining macron]](https://www.rsc.org/images/entities/char_0033_0304.gif) c, a =
b = 18.328(9), c = 48.80(2) Å, γ = 120°,
V = 14196(11) Å3, Z = 6,
Dc = 1.596 g cm−3. The asymmetric unit
is 1/6 of the molecular entity as defined above with Fe, the tripodal N
atom and Mn, located in special positions (symmetry 3, 3 and 2). A blue
dark prism single crystal (0.2 × 0.3 × 0.5 mm) was mounted on
an Enraf-Nonius CAD4 diffractometer with graphite monochromated Mo-Kα
radiation (λ = 0.7107 Å). The unit cell dimensions were
refined from setting angles of 25 reflections (6 < θ <
9°). The data collection was via the θ–2θ
scan technique mode (range 2–25.8°). Three standard reflections
were measured every hour and showed no significant decay. A total of 7674
reflections was collected (−22 ⩽
h
⩽ 19, 0 ⩽
k
⩽ 22, 0 ⩽
l
⩽ 54). From 2294 independent
reflections, 1279 were considered with [I >
2ς(I)]. Lorentz-polarisation and absorption corrections
were applied. The structure was solved by direct methods (program SHELXS86)
and refined on F2 for all reflections by the
least-squares method using SHELXL-93. Hydrogen atoms were included in the
refinement at their ideal positions with an isotropic thermal parameter 1.2
times that of the bonded atoms. The crystal structure is affected by
disorder. Two different sites of each CF3 group were found from
difference Fourier syntheses. Best convergence was obtained with occupancy
factors of 60 and 40%. Refinement was performed with constraints. The final
conventional residuals are R1 = 0.054 and wR2 = 0.14 for
[I > 2ς(I)] and R1 = 0.11 and
wR2 = 0.19 (all data). CCDC 182/1490. See http:// www.rsc.org/suppdata/cc/a9/a907068k/ for crystallographic files in .cif
format. c, a =
b = 18.328(9), c = 48.80(2) Å, γ = 120°,
V = 14196(11) Å3, Z = 6,
Dc = 1.596 g cm−3. The asymmetric unit
is 1/6 of the molecular entity as defined above with Fe, the tripodal N
atom and Mn, located in special positions (symmetry 3, 3 and 2). A blue
dark prism single crystal (0.2 × 0.3 × 0.5 mm) was mounted on
an Enraf-Nonius CAD4 diffractometer with graphite monochromated Mo-Kα
radiation (λ = 0.7107 Å). The unit cell dimensions were
refined from setting angles of 25 reflections (6 < θ <
9°). The data collection was via the θ–2θ
scan technique mode (range 2–25.8°). Three standard reflections
were measured every hour and showed no significant decay. A total of 7674
reflections was collected (−22 ⩽
h
⩽ 19, 0 ⩽
k
⩽ 22, 0 ⩽
l
⩽ 54). From 2294 independent
reflections, 1279 were considered with [I >
2ς(I)]. Lorentz-polarisation and absorption corrections
were applied. The structure was solved by direct methods (program SHELXS86)
and refined on F2 for all reflections by the
least-squares method using SHELXL-93. Hydrogen atoms were included in the
refinement at their ideal positions with an isotropic thermal parameter 1.2
times that of the bonded atoms. The crystal structure is affected by
disorder. Two different sites of each CF3 group were found from
difference Fourier syntheses. Best convergence was obtained with occupancy
factors of 60 and 40%. Refinement was performed with constraints. The final
conventional residuals are R1 = 0.054 and wR2 = 0.14 for
[I > 2ς(I)] and R1 = 0.11 and
wR2 = 0.19 (all data). CCDC 182/1490. See http:// www.rsc.org/suppdata/cc/a9/a907068k/ for crystallographic files in .cif
format. |
| § Sample elemental analysis is in agreement with the proposed formula while the IR spectrum of the polymer is the superimposition of the spectra of iron and manganese building blocks. This is consistent with the geometric similarity of the Fe unit in the monomer and in the polymer (X-ray structure). |
| ¶ Magnetic properties have been recorded for ground crystals from the same batch used for X-ray diffraction and elemental analysis. |
| || At 300 K, χMT = {[0.125gFe2S(S + 1)] × 2} + {[0.125gMn2S(S + 1)] × 3}. A gFe-value of 2.28 is consistent with the gFe value obtained for the iron monomer (2.35).4 |
| ** This is entirely consistent with imidazolate bridges which are known to induce only very weak magnetic interactions.1 |
| This journal is © The Royal Society of Chemistry 2000 |