The mutagenic mechanism of oxygenated alkylhydrazones occurs through alkyl radicals and alkyldiazonium ions†
Abstract
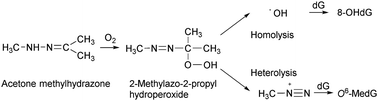
Acetone alkylhydrazones have been reported to be mutagenic in Salmonella typhimurium TA1535 after exposure to oxygen, and the corresponding 2-alkylazo-2-propyl hydroperoxides are formed by autoxidation as a result. The aims of this study were to investigate the mutagenic mechanisms of a methyl analogue, 2-methylazo-2-propyl hydroperoxide (MAPH), by comparing the mutagenic potency of specific Salmonella strains, detecting the DNA adducts that cause mutagenicity, and observing the hydroxyl radical and methyl radical with the electron spin resonance (ESR) spin-trapping method. MAPH showed stronger mutagenicity in both Salmonella typhimurium YG3001, a strain sensitive to hydroxyl radicals, and Salmonella typhimurium YG7108, a strain sensitive to alkylating agents, than the original Salmonella typhimurium TA1535 strain. Moreover, MAPH resulted in the formation of 8-hydroxy-2′-deoxyguanosine and O6-methyl-2′-deoxyguanosine in a reaction with DNA. These results showed that the mutagenicity of hydrazones was ascribed to the generation of reactive species by autoxidation, namely that of the alkyldiazonium ion and also the hydroxyl radical.


 Please wait while we load your content...
Please wait while we load your content...