Effect of base sequence context on the conformational heterogeneity of aristolactam-I adducted DNA: structural and energetic insights into sequence-dependent repair and mutagenicity†
Abstract
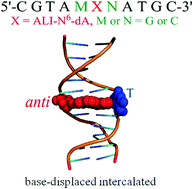
Aristolochic acids (AAs) are nephrotoxic and potentially carcinogenic plant mutagens that form bulky DNA adducts at the exocyclic amino groups of the purines. The present work utilizes classical molecular dynamics simulations and free energy calculations to investigate the role of lesion site sequence context in dictating the conformational outcomes of DNA containing ALI-N6-dA, the most persistent and mutagenic adduct arising from the AAs. Our calculations reveal that the anti base-displaced intercalated conformer is the lowest energy conformer of damaged DNA in all sequence contexts considered (CXC, CXG, GXC and GXG). However, the experimentally-observed greater mutagenicity of the adduct in the CXG sequence context does not correlate with the relative thermodynamic stability of the adduct in different sequences. Instead, AL-N6-dA adducted DNA is least distorted in the CXG sequence context, which points toward a possible differential repair propensity of the lesion in different sequences. Nevertheless, the structural deviations between adducted DNA with different lesion site sequences are small, and therefore other factors (such as interactions between the adducted DNA and lesion-bypass polymerases during replication) are likely more important for dictating the observed sequence-dependent mutagenicity of ALI-N6-dA.

 Please wait while we load your content...
Please wait while we load your content...