Screening and validation for plasma biomarkers of nephrotoxicity based on metabolomics in male rats†
Abstract
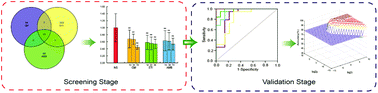
Currently, drug-induced nephrotoxicity is widespread and seriously affects human health. However, the conventional indexes of renal function lack sensitivity, leading to a delay in the detection of nephrotoxicity. Therefore, we need to identify more sensitive indexes for evaluating nephrotoxicity. In this study, we used gentamicin (100 mg kg−1), etimicin (100 mg kg−1) and amphotericin B (4 mg kg−1) to establish renal injury models in rats, and we collected information using ultra-performance liquid chromatography quadrupole time-of-flight mass spectrometry in the screening stage. Thirteen nephrotoxicity metabolites were selected after multivariate statistical and integration analyses. Then, we conducted trend analysis to select 5 nephrotoxicity biomarkers [thymidine, LysoPC(16:1), LysoPC(18:4), LysoPC(20:5), and LysoPC(22:5)] whose content changed consistently at different timepoints after drug administration. To verify the sensitivity and specificity of these biomarkers for nephrotoxicity, receiver operating characteristic (ROC) and support vector machine (SVM) analyses were applied. The area under the curve of the 5 biomarkers were 0.806–0.901 at the 95% confidence interval according to the ROC analysis. We used the SVM classified model to verify these biomarkers, and the prediction rate was 95.83%. Therefore, the 5 biomarkers have strong sensitivity and high accuracy; these biomarkers are more sensitive indexes for evaluating renal function to identify nephrotoxicity and initiate prompt treatment.

 Please wait while we load your content...
Please wait while we load your content...