Novel and asymmetric S,N-heterocyclics with fused six-membered rings for organic field effect transistor applications†
Abstract
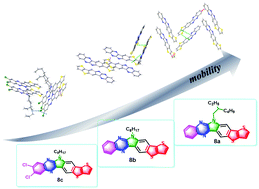
We herein describe the design, synthesis, characterization and property evaluation of three novel π-conjugated ladder-type and asymmetric S,N-heterocyclics with fused six-membered rings. The unique molecular design incorporates electron-deficient pyrazine and electron-donating thienothiophene in one molecular skeleton to achieve fused and asymmetric heterocyclics with six consecutive rings, and we tuned the molecular structure with different alkyl chains and the ending chlorine substitution. Single-crystal structure studies and analysis of opto-electronic properties were carried out to understand the structure–property relationships for applying these π-scaffolds in organic single-crystal transistors. Charge mobility studies reveal a promising hole mobility of 0.64 cm2 V−1 s−1 for one of the asymmetric S,N-heterocyclics.


 Please wait while we load your content...
Please wait while we load your content...