Dynamic investigation of gas-releasing chemical reactions through a photonic crystal†
Abstract
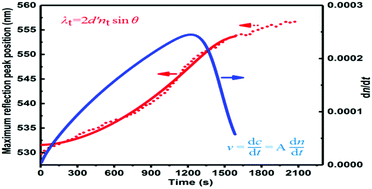
A new strategy for real-time, non-destructive and dynamic detection has been demonstrated to monitor the process and kinetics of a chemical reaction using a photonic crystal (PC). The chemical balance time, reaction rate and process of microchemical reactions of H2O2 with KI, the Friedel–Crafts reaction and Fenton oxidative degradation reaction, were monitored through the dynamic stopband shift of the PC under different reaction conditions. The chemical reaction endpoint was determined easily, and similar reactions could be distinguished through obvious differences in balance time and reaction rate, and optimal reaction conditions were determined using this PC method. The chemical reaction rate and average activation energy were calculated from the stopband shift curve of the PC with time. The chemical reaction mechanism was also investigated and analyzed according to the stopband shift curve of the PC with time. This novel chemical kinetic detection method based on a PC is universal and has great potential for use in monitoring other chemical reactions.


 Please wait while we load your content...
Please wait while we load your content...