Two-phase anion exchange synthesis: multiple passivation for highly efficient and stable CsPbCl3 nanocrystals†
Abstract
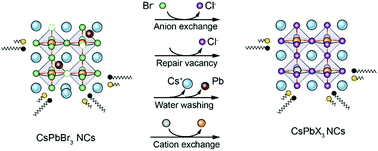
In recent years, substantial progress has been made in the synthesis of colloidal CsPbX3 (X = Cl, Br, and I) nanocrystals (NCs). However, the anion exchange reaction always synthesizes CsPbCl3 NCs with low efficiency and poor stability. Here, first, through separating the halide precursors, mainly metal halide salts as CdCl2 in water, we propose a two-phase anion exchange method, successfully preparing CsPbCl3 NCs with a well-maintained cubic morphology and high photoluminescence quantum yields of about 95%. A controllable Cl-to-Br exchange rate is readily achieved by controlling the reaction time or the Cl− ion concentration. Benefiting from the good solubility of CdCl2 in water, Cl− ions are provided continuously, guaranteeing an adequate Cl-to-Br exchange at a high concentration of the original substrate. Rich Cl− ions, water and even the Cd2+ ions perform effective passivation of the nonradiative recombination centers likely stemming from Cl vacancies or other impurities, which has been confirmed by the transient optical spectroscopy measurements and elemental characterization. Also, the CsPbCl3 NCs reveal improved air stability and excellent photostability. We believe that the two-phase anion exchange method offers a new way to obtain high-efficiency CsPbCl3 NCs through a postsynthetic chemical transformation route, and helps to promote further corresponding applications in optoelectronic devices.


 Please wait while we load your content...
Please wait while we load your content...