An insight into de Vries behaviour of smectic liquid crystals from atomistic molecular dynamics simulations†
Abstract
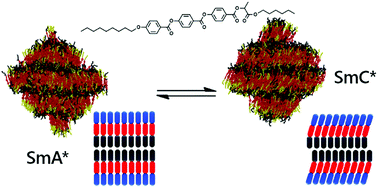
Fully atomistic molecular dynamics simulations have been performed on the ferroelectric liquid crystal compound 9HL, which exhibits de Vries character. Simulations were carried out at a range of temperatures, and both SmA* and SmC* phases were successfully simulated. Experimental trends in orientational order parameter, translational order parameter, layer spacing, and tilt angle were found to be matched by the simulations. The simulations were analysed in the context of multiple reported models developed from the interpretation of experimental data, none of which were found to individually describe the simulated behaviour. Further analysis demonstrated the formation of distinct aromatic and lactate sub-layers in the 9HL simulations. The aromatic sub-layers exhibited tilting behaviour at the SmA*/SmC* transition, whereas alignment within the lactate sub-layers was found to be relatively unchanged, maintaining a SmA-like configuration even in the SmC* phase. This behaviour provides a simple explanation for de Vries behaviour, where only a fraction of the layer structure exhibits an overall tilt in the SmC phase.


 Please wait while we load your content...
Please wait while we load your content...