A phenyl-removal strategy for accessing an efficient dual-state emitter in the red/NIR region guided by TDDFT calculations†
Abstract
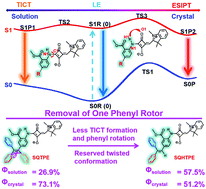
The development of molecules with efficient dual-state emission in the red/NIR region is significant for their broader application prospects in the biological field, but still remains a great challenge. Guided by in-depth TDDFT calculations, herein we firstly demonstrate a phenyl-removing strategy in TPE-merged squaraine dyes for accessing intense red/NIR emissions in both solution and crystalline states, on the basis of fully decoding the bidirectional TICT/ESIPT mechanism. By removing one phenyl rotor in the TPE segment, both less TICT formation and phenyl rotations facilitate the brightest red emission (λem = 586/622 nm, ΦPL = 57.5%) of SQHTPE in solution, while the reserved hydrogen-bonding packing and the ESIPT process guarantee the intense red/NIR emission (λem = 670 nm, ΦPL = 51.2%) in its crystalline state. Moreover, efficient red bioimaging in HUVECs has been achieved via naked SQHTPE, which provides a new vision for directly applying squaraine dyes in biological applications.


 Please wait while we load your content...
Please wait while we load your content...