Constructing stable phenalenyl-based neutral radicals: a theoretical study†
Abstract
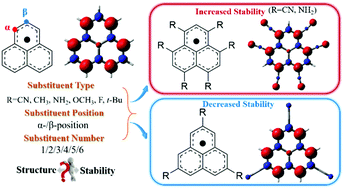
Organic radicals with unpaired electrons have attracted tremendous attention recently, but it remains a great challenge for the rational design of stable radicals due to the lack of clear structure-stability relationships and reliable molecular construction guidelines. In this work, we perform a systematic computational investigation of phenalenyl-based radicals to elucidate the structure–property relations between the substitution position, number and type, and the thermodynamic stability of the stable radicals. The results indicate that the substituents at the α-position can improve the stability of phenalenyl-based radicals, and the π-conjugated groups (such as –CN) and the lone-pair groups with small electronegativity (such as –NH2) show the best stabilization effects. Excitingly, 47 radicals with improved thermodynamic stability compared to the phenalenyl radical were revealed, which should be highly attractive for experimental investigations. These findings with rich physical insights on the structure-stability relation of phenalenyl-based radicals should provide important clues for designing stable organic radicals.


 Please wait while we load your content...
Please wait while we load your content...