Bright mechanoluminescent luminogens even in daylight through close intermolecular interaction with the characteristic of hybridized local and charge transfer (HLCT)†
Abstract
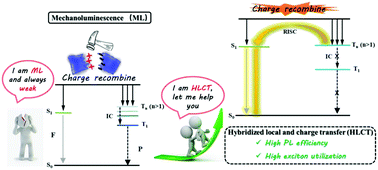
The study of mechanoluminescence (ML) has attracted much attention for its widespread applications. Until now, organic ML luminogens are still very scarce, especially those with bright emissions. Herein, three donor–acceptor (D–A) molecules with triphenylamine, phenothiazine and phenoxazine as electron donors and p-fluorophenylcarbonyl as an electron acceptor are designed and synthesized via a facile approach with the Friedel–Crafts reaction in one step, named FCO-TPA, FCO-CzS and FCO-CzO, which exhibit high emission quantum yields both in solution and solid states. The fluorescence solvatochromic experiments and theoretical calculations prove their unique HLCT state characteristics, which can largely enhance the excited state energy utilization. Accordingly, very bright mechanoluminescence (ML) even in daylight is achieved in FCO-TPA with non-centrosymmetric molecular arrangement and close intermolecular interactions.
- This article is part of the themed collection: Journal of Materials Chemistry C Emerging Investigators


 Please wait while we load your content...
Please wait while we load your content...