How organic ligands affect the phase transition and fluorescent stability of perovskite nanocrystals†
Abstract
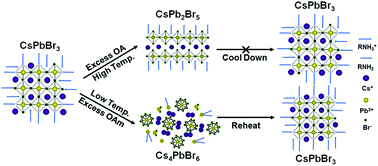
It is well known that the surface ligand systems composed of aliphatic carboxylic acids and amines are very important to the synthesis of perovskite nanocrystals (NCs), which have drawn wide attention in recent years due to their excellent photoelectric performances. However, the effect of individual acids and amines on the crystal growth and optical properties of this kind of NCs remains poorly understood. In this work, we have demonstrated that these ligands play a role like a double-sided sword in the assembly of NCs. Not only could they promote the formation of NCs, but also the excess oleylamine (OAm) would induce the decomposition of NCs and the excess oleic acid (OA) would induce the phase transformation of the NCs from cubic CsPbBr3 to tetragonal CsPb2Br5 gradually, along with a dramatic decrease of photoluminescence. Interestingly, the decomposed structure and faded PL of the NCs induced by excess OAm could be recovered by further thermal treatment, which is probably attributed to the thermodynamic equilibrium between OAm and Br− with Pb2+. These results provide insights into the growth and transition of perovskite NCs with different crystal phases and their optical properties.


 Please wait while we load your content...
Please wait while we load your content...