High-stability fluorescent perovskites embedded in PbBrOH triggered by imidazole derivatives in water†
Abstract
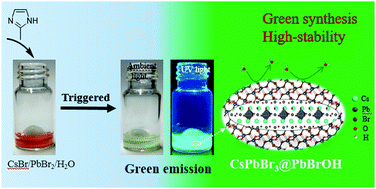
The optoelectronic application of fluorescent lead halide perovskite (LHP) nanocrystals is limited by their poor stability in humid atmosphere. However, we found that high-quality and high-stability fluorescent perovskites embedded in PbBrOH can be synthesized via the reaction of PbBr2 with MBr (M: Cs or CH3NH3+) triggered with imidazole derivatives in water. Imidazole can etch and hydrolyze the nonluminescent MPbBr3 to form high-quality fluorescent perovskites. After the perovskites are formed, a protective PbBrOH matrix grows rapidly on the surface of the perovskite nanocrystals and acts as a water-resistant layer. The embedded fluorescent perovskites can exhibit great stability even in water, heat and UV light with a PLQY of up to 30%. Density functional theory (DFT) calculations show that the enhanced stability results from the positive energy cost of the water molecule entering the lattice of PbBrOH, which is much higher than the negative value for water entering CsPbBr3; therefore, water is prevented by the PbBrOH surface from entering the embedded fluorescent perovskite. The PbBrOH surface layer has twofold beneficial effects: (i) preventing water from entering the perovskite nanocrystals, (ii) dispersing the perovskite nanocrystals in the matrix and preventing their aggregation so that highly luminescent composites can be formed. Our strategy opens a new avenue for the development of high-efficiency and high-stability fluorescent inorganic and organic perovskites.


 Please wait while we load your content...
Please wait while we load your content...