Color switching in V3O7·H2O films cycled in Li and Na based electrolytes: novel vanadium oxide based electrochromic materials†
Abstract
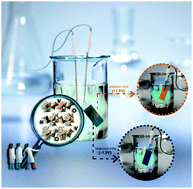
Vanadium oxides exhibiting various V/O stoichiometries are considered as one of the most promising chromogenic materials for energy purposes. Commonly addressed are the thermochromic properties of VO2 and the electrochromic ones of V2O5. In this work, we report a robust synthetic methodology that provides access to stoichiometry-control of the final powder namely, a hydrothermal route is used to synthesize pure vanadium oxyhydroxide with mixed valence states (V5+ and V4+) of the V3O7·H2O (H2V3O8) formula. Upon synthesis of the powder, the influence of reducing agents on the structure, morphology and crystallite size is investigated. The electrochromic properties of the doctor bladed V3O7·H2O thick film (≈ 1.2 µm) in Li- and Na-based electrolytes show in both cases good reversibility and good color switching in between reduced and oxidized states. Preliminary investigations of the EC mechanism allow the identification of faradaic and capacitive contributions in this oxide showing switching times of only a few seconds.


 Please wait while we load your content...
Please wait while we load your content...