Effect of the phase structure on the catalytic activity of MoO3 and potential application for indoor clearance†
Abstract
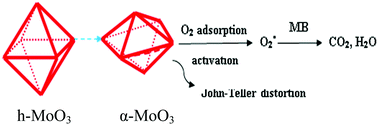
In this work, α-MoO3 and h-MoO3 are synthesized by a simple hydrothermal method. Under ambient conditions, α-MoO3 has a high catalytic wet air oxidation (CWAO) activity for methylene blue (MB), while h-MoO3 has no activity: 64% of MB can be degraded after 20 min. When adding an additional man-made light source, the performances of h-MoO3 and α-MoO3 remain almost unchanged. However, with elevating the reaction temperature from 25 to 40 °C, the activity has not been improved for h-MoO3, while the activity of α-MoO3 also has no improvement from 25 to 35 °C. When the temperature rises to 40 °C, the performance of α-MoO3 improved significantly: the degradation percentage reaches 90% after 20 min. Crystal structure analysis results show that the activity of α-MoO3 originates from Jahn–Teller distortion of the [MoO6] octahedron, resulting in the formation of Lewis acid sites. Furthermore, H2-temperature programmed reduction (H2-TPR) and O2-temperature programmed desorption (O2-TPD) results indicate that α-MoO3 has a higher lattice oxygen mobility and a higher oxidization ability than h-MoO3. This ambient CWAO process is energy-saving and low-cost, which is promising for indoor clearance.


 Please wait while we load your content...
Please wait while we load your content...