Tuning the surface charge density of exfoliated thin molybdenum disulfide sheets via non-covalent functionalization for promoting hydrogen evolution reaction†
Abstract
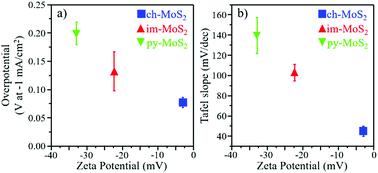
The scalable and facile generation of hydrogen fuel via water splitting requires robust and cost-effective earth-abundant electrocatalysts for hydrogen evolution reaction (HER). We report for the first time that the surface charge density of exfoliated monolayer molybdenum disulfide (MoS2) sheets can be successfully tuned with the assistance of exfoliants, such as pyridinium tribromide (py), imidazole (im), and chlorophyll (ch), and thereby significantly influence the kinetics of the HER. The surface charge potential of the exfoliated monolayer py-MoS2, im-MoS2, and ch-MoS2 sheets are −33 mV, −22 mV and −2 mV, respectively. As the surface charge potential approaches zero, the Tafel slope required varies from 140 mV dec−1 for py-MoS2 to 45 mV dec−1 for ch-MoS2. Moreover, to achieve real-world applications, the exfoliated thin ch-MoS2 sheets were fabricated to form flexible composite papers, and they exhibited excellent electrocatalytic activity with further enhanced Tafel slope of 35 mV dec−1, which is nearly identical to that of bulk Pt, “the gold standard” for HER, an ultra-low onset potential of −42 mV and over 100 h of electrochemical durability. This convenient non-covalent functionalization method provides opportunities for many other applications of exfoliated thin MoS2 sheets and other two-dimensional transition metal dichalcogenides (2D-TMDCs).


 Please wait while we load your content...
Please wait while we load your content...