Uncovering the microscopic mechanism of incorporating Mn2+ ions into CsPbCl3 crystal lattice†
Abstract
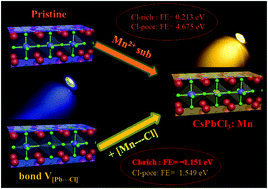
Cation doping strategy has been extensively employed to enhance the charge carrier mobility and improve the stability of halide perovskites; however, the fundamental understanding of the inherent doping mechanism is still a challenge. Herein, based on a unique four-precursor synthetic strategy and density functional theory (DFT) calculation results, we verify that excessive chloride ion concentration benefits the formation of the bond [Pb⋯Cl] vacancy pair as well as the subsequent incorporation of the [Mn⋯Cl] ion pair synchronously. As a result, high Cl− ion concentration greatly promotes the incorporation of the Mn2+ ions into the CsPbCl3 crystal lattice. The particle size reduction of the CsPbCl3 perovskite with the incorporation of the Mn2+ ions is attributed to the increased surface electron charge density, which inhibits the diffusion of the negatively charged Cl− ions to the nuclei surface. These deep insights into the inherent Mn2+ doping mechanism could guide the realization of novel ion-doped perovskites with new optoelectronic performances.


 Please wait while we load your content...
Please wait while we load your content...