Efficient thermally activated delayed fluorescence based on carbonitrile-substituted pyridine and carbazole†
Abstract
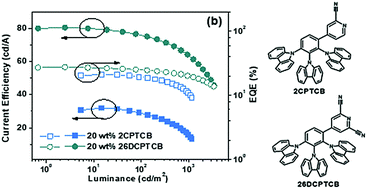
Efficient thermally activated delayed fluorescence (TADF) emitters are promising for future applications in organic light-emitting diodes (OLEDs). In this study, two carbonitrile-substituted pyridine and carbazole based light-emitting molecules with a compact architecture were designed and synthesized. Although they have the same donor units, their emission color can be tuned by varying the electron withdrawing capacity of the acceptor. Thanks to the large torsion angle between the benzene core and each donor/acceptor, the lowest unoccupied molecular orbitals (LUMOs) and the highest occupied molecular orbitals (HOMOs) of these compounds are separately distributed on the carbonitrile-substituted pyridine and carbazole units, respectively. Their small energy gap between the singlet and triplet states, as well as typical TADF feature were characterized. In addition, an OLED device based on these CN-substituted pyridine compounds gave external quantum efficiencies of up to 21.1% and 27.6% for blue and green light, respectively.


 Please wait while we load your content...
Please wait while we load your content...