Synthesis of an isomerically pure thienoquinoid for unipolar n-type conjugated polymers: effect of backbone curvature on charge transport performance†
Abstract
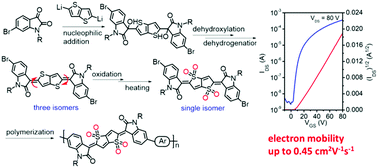
A single-isomer of the thienoquinoidal unit, IDOTT, has been synthesized via a new synthetic route involving regioselective nucleophilic addition, dihydroxylation, dehydrogenation, oxidation and isomerization, and the structure of IDOTT was unambiguously confirmed by X-ray crystallographic analysis. Compared with the reported synthetic route, this newly developed strategy possessed a wide range of substrate applicability. Moreover, IDOTT showed good air stability and excellent compatibility to chemical reactions, endowing the potential to construct conjugated polymers by different cross-coupling reactions. With IDOTT as the acceptor unit, three donor–acceptor (D–A) conjugated polymers, i.e. PIDOTT-T, PIDOTT-TT and PIDOTT-BT, were synthesized by Stille polycondensation. These three polymers showed deep highest-occupied molecular orbital (HOMO) (<−5.90 eV) and lowest unoccupied molecular orbital (LUMO) (∼−4.04 eV) energy levels, and exhibited unipolar n-type behavior in organic thin-film transistors (OTFTs). Among these polymers, PIDOTT-BT delivered the best device performance with an electron mobility of up to 0.45 cm2 V−1 s−1, which is the highest for n-type conjugated polymers with quinoidal units. The superior performance of PIDOTT-BT can be attributed to its highly ordered thin-film packing that stemmed from the small backbone curvature.


 Please wait while we load your content...
Please wait while we load your content...