Impact of new skeletal isomerization in polymer semiconductors†
Abstract
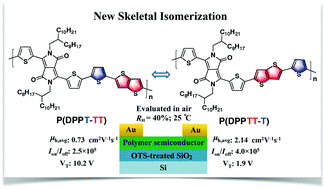
Two novel isomeric polymers P(DPPT-TT) and P(DPPTT-T) based on thiophene-flanked diketopyrrolopyrrole, thieno[3,2-b]thiophene and thiophene building units were designed and synthesized via a new skeletal isomerization strategy. This study emphasizes on the impact of this skeletal isomerization on the photoelectric properties and organic field-effect transistor (OFET) performances of the resulting isomeric polymers. Both polymers show similar light absorption properties and excellent thermostability and have a comparable molecular weight. They also display typical p-type characteristics in air-tested OFETs. However, P(DPPTT-T) exhibits significantly better OFET performances. In particular, its average hole mobility reaches 2.14 cm2 V−1 s−1, triple that of P(DPPT-TT). The higher mobility of P(DPPTT-T) might be attributed to the interconnected, smooth and thicker nanofibrillar network morphology and the relatively higher crystallinity of its thin films, and its conjugated chains possess a smaller π–π stacking distance.


 Please wait while we load your content...
Please wait while we load your content...