Electron affinity of boron-terminated diamond (001) surfaces: a density functional theory study
Abstract
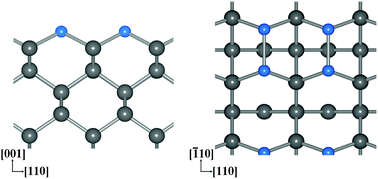
Boron-terminated bare and oxidized diamond (001) surfaces are first proposed in this paper and are modelled by adsorbing the boron (B) atoms onto the bare and oxidized diamond (001) surfaces, respectively. The electron affinities (EAs) and adsorption energies per B atom of the B-terminated bare and oxidized diamond (001) surfaces were investigated via density functional theory calculations. Applying 1 monolayer (ML) B coverage yields a more negative EA (NEA) than 0.25 ML or 0.5 ML coverage on the bare diamond surfaces, and a larger positive EA (PEA) on the oxidized diamond surfaces. Their positive adsorption energies mean that 1 ML B coverage is energetically favorable on both the bare and oxidized diamond (001) surfaces. The B-terminated bare diamond (001) surfaces with 1 ML coverage have an NEA of up to −1.39 eV, and their adsorption energy is higher than that of hydrogen-terminated diamond (001) surfaces by 1.49 eV, thus they could potentially be applied to create thermally stable electron emission devices. The B-terminated oxidized diamond (001) surfaces (1 ML coverage) have a PEA of up to 3.21 eV, larger than the EAs of ether oxidized (2.39 eV), nitrogen-terminated (3.01 eV), or fluorine-terminated (2.34 eV) diamond (001) surfaces. Consequently, the B-terminated oxidized diamond (001) surfaces show great potential for NV and SiV quantum sensing applications.


 Please wait while we load your content...
Please wait while we load your content...