Fluorination of the tetraphenylethene core: synthesis, aggregation-induced emission, reversible mechanofluorochromism and thermofluorochromism of fluorinated tetraphenylethene derivatives†
Abstract
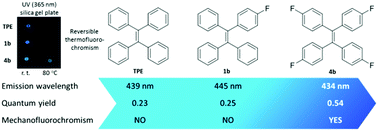
The Suzuki–Miyaura cross-coupling reactions of bromoalkenes with fluorophenylboronic acids afforded 17 fluorinated tetraphenylethene (FTPE) compounds that have only fluorine substituent(s) directly on the tetraphenylethene (TPE) core with different numbers and substitution positions of the fluorine atom(s). The X-ray structures of four FTPEs show that these FTPE derivatives have C–H⋯F and C–H⋯π hydrogen bonds in the crystal packing. DFT calculations indicate that both the HOMOs and LUMOs of the FTPEs essentially have lower energy levels than those of the parent TPE, and the energy gaps are highly dependent on the substitution pattern. These FTPEs exhibit aggregation-induced emission characteristics similar to most TPE derivatives but the aggregation process was found to be gradual and time-dependent. The emission wavelengths of both aggregates from tetrahydrofuran/H2O and solids, and the corresponding emission quantum yields of the solid samples vary with different substitution patterns. In addition, two examples of these FTPEs show reversible mechanofluorochromic and thermofluorochromic properties (the latter was only shown on silica gel), while the parent TPE does not exhibit mechanochromism under the same conditions.


 Please wait while we load your content...
Please wait while we load your content...