Pure E/Z isomers of N-methylpyrrole-benzohydrazide-based BF2 complexes: remarkable aggregation-, crystallization-induced emission switching properties and application in sensing intracellular pH microenvironment†
Abstract
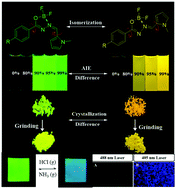
Organic fluorescent molecules with E/Z isomers usually have different photophysical properties owing to their disparate configurations, which lead to differences in molecular polarity and the chemical environment of atoms. However, due to the difficulty in the synthesis of their pure E and Z conformers, very limited research efforts have been devoted to studying the different photophysical properties of these isomers. In this work, two pairs of pure E/Z isomers of novel fluoroboron acylhydrozone dyes based on 2,2-difluoro-3-[(N-methylpyrrol-2-yl)methylene]-5-phenyl-1,3,4,2-oxadiazaborole (MPOAB) with two different electronic substituent groups (OMe or NEt2) were developed for the first time. These isomers were each characterized by NMR, X-ray structure analysis, HRMS, and absorption and PL spectroscopy. All isomers showed weak fluorescence properties in organic solvents and strong fluorescence in the solid state as well as excellent aggregation-induced emission (AIE) properties. More interestingly, the photophysical properties of the E/Z isomers, especially their aggregation and crystallization-induced emission, are significantly different. These results indicate that the AIE properties of these compounds are mainly attributed to the restriction of single bond rotations between the two aromatic rings and the five-membered fluoroboric ring, while participation of E/Z isomerization is finite. Besides, their photoisomerization, grinding, viscosity, and acid–base fluorescence response properties were also studied. In addition, an isomer of them, as an example, was successfully applied for analyzing the intracellular pH microenvironment of living cancer cells.


 Please wait while we load your content...
Please wait while we load your content...