Dibenzofuran/dibenzothiophene as the secondary electron-donors for highly efficient blue thermally activated delayed fluorescence emitters†
Abstract
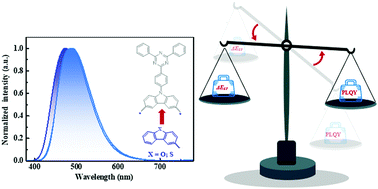
By introducing two novel electron-donating (D) moieties dibenzothiophene (DBT) and dibenzofuran (DBF) as the secondary D groups, we designed and synthesized four novel blue thermally activated delayed fluorescence (TADF) emitters DBTCz-Trz, DBFCz-Trz, BDBTCz-Trz and BDBFCz-Trz. The secondary DBF and DBT segments all significantly participated in the highest occupied molecular orbital (HOMO) delocalization, resulting in effective TADF characteristics and high photoluminescence quantum yields simultaneously for all four emitters. In the devices, DBTCz-Trz and DBFCz-Trz successfully exhibit identical blue emission with a peak at 472 nm and a CIE coordinate of (0.17, 0.28) in the devices, while BDBTCz-Trz and BDBFCz-Trz exhibit cyan emissions. Moreover, the maximum forward-viewing external quantum efficiencies are determined to be as high as 21.7% for DBTCz-Trz, 21.6% for DBFCz-Trz, 23.4% for BDBTCz-Trz, and 25.1% for BDBFCz-Trz, respectively. These results not only prove the great potential of amine-free electron-donating components such as DBF and DBT in constructing TADF emitters for electroluminescence, but they also reveal the importance of extending the frontier orbital molecular distributions for TADF molecular optimization.


 Please wait while we load your content...
Please wait while we load your content...