Solution-processable, high luminance deep-blue organic light emitting devices based on novel naphthalene bridged bis-triphenylamine derivatives†
Abstract
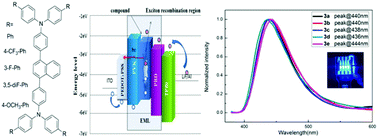
A series of naphthalene bridged bis-triphenylamine derivatives with a twisted structure was designed, synthesized and characterized. The dependence of their thermal, photophysical and electrochemical properties and performance as emitters in OLEDs on their chemical structure was systematically studied by the introduction of aryl groups with electron-donating or electron-withdrawing substituents to the naphthalene bridged bis-triphenylamine core. These compounds exhibited steady blue light emissions and high Td values ranging from 468 to 500 °C. Most importantly, the deep-blue OLEDs were successfully fabricated using a solution processed method by blending PVK and PBD to improve OLED performance. The optimal device E exhibited a very high luminance of 10 407 cd m−2 and a maximum current efficiency of 7.80 cd A−1 with CIE coordinates of (0.166, 0.097). These results indicated that these compounds with the twisted naphthalene bridged bis-triphenylamine core could show stable deep-blue electroluminescence properties and introducing the electron-donating group (–OCH3) could enable high luminance and current efficiency for OLEDs.


 Please wait while we load your content...
Please wait while we load your content...