Programmed twisting of phenylene–ethynylene linkages from aromatic stacking interactions†
Abstract
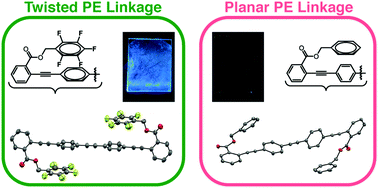
Control over the conformation and packing of conjugated materials is an unsolved problem that prevents the rational design of organic optoelectronics, such as preventing self-quenching of luminescent molecules. Exacerbating this challenge is a general lack of widely applicable strategies for controlling packing with discrete, directional non-covalent interactions. Here, we present a series of conjugated molecules with diverse backbones of three or four arenes that feature pentafluorobenzyl ester substituents. Nearly all the compounds reveal intramolecular stacking interactions between the fluoroarene (ArF) side-chains and non-fluorinated arenes (ArH) in the middle of the chromophores; a twisted PE linkage accompanies each example of this intramolecular ArF–ArH stacking. Furthermore, these molecules can resist dramatic changes to emission upon transition from organic solution to thin film when ArF rings prevent interchromophore interactions. By broadening the structural space of conjugated backbones over which ArF–ArH stacking can twist PE linkages reliably and prevent self-quenching of solids with simple synthetic approaches, this work suggests fluorinated benzyl ester substituents adjacent to phenylene ethynylene linkages as supramolecular synthons for the crystal engineering of organic optoelectronic materials.


 Please wait while we load your content...
Please wait while we load your content...
