Effect of end group functionalisation of small molecules featuring the fluorene–thiophene–benzothiadiazole motif as emitters in solution-processed red and orange organic light-emitting diodes†
Abstract
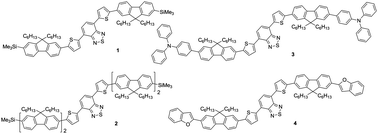
A series of red fluorescent materials (compounds 1–4), which each contain the symmetric fluorene–thiophene–BT–thiophene–fluorene core, is presented along with their performance in solution-processed OLED devices. Extending the molecular conjugation through end-capping with additional fluorene units (compound 2), or through incorporation of donor functionalities (compounds 3 and 4) improves OLED performance relative to the parent compound 1. Notably, incorporating triphenylamine donor groups in compound 3 led to solution-processed OLED devices operating with a peak luminance of 2888 cd m−2 and a low turn-on voltage (3.6 V).
- This article is part of the themed collection: 10th Anniversary: Dedicated Authors


 Please wait while we load your content...
Please wait while we load your content...