Smartly designed AIE triazoliums as unique targeting fluorescence tags for sulfonic biomacromolecule recognition via ‘electrostatic locking’†
Abstract
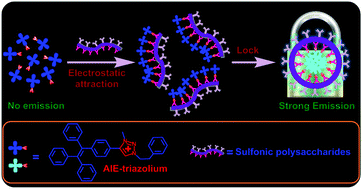
Specific electrostatic interactions are highly efficient for fluorescent bioassays but they are difficult to achieve. In this work, smartly designed aggregation-induced emission (AIE) triazoliums with donor–acceptor structures were developed via click conjugating a triazole heterocycle onto a tetraphenylethylene (TPE) fluorophore followed by a cationization reaction. The strongly electron-deficient nature of the triazolium coupled with intermolecular electrostatic repulsions resulted in the decrease of TPE's AIE character when it was in solution. However, specific “electrostatic locking” was found to take place between the triazolium and multiple sulfonates and this unique targeting combination attenuated triazolium's electrophilicity and electrostatic repulsion resulting in the recovery of the AIE. Cryo-electron microscopy (Cryo-EM) was used to reveal the in situ aggregation state of the probe. The triazolium probes exhibited a highly specific turn-on fluorescence response toward sulfonic biomacromolecules such as heparin, chondroitin and sulfonate cyclodextrin, demonstrating that the AIE-triazoliums have great potential for bioassay applications.


 Please wait while we load your content...
Please wait while we load your content...