Structure–property relationship in multi-stimuli responsive D–A–A′ benzothiazole functionalized isomers†
Abstract
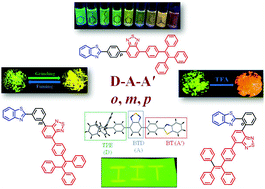
Multichromophoric D–A–A′ molecules comprising of benzothiazole (BT) as A′, benzothiadiazole (BTD) as A and tetraphenylethylene (TPE) as D were designed and synthesized as positional isomers p-BT, m-BT and o-BT by attaching the BTD–TPE moiety ortho, meta and para to the phenyl BT unit. The positional change exploited in these isomers can influence the acceptor strength and molecular packing. Hence, a comparative study of the photophysical and electronic properties has been carried out to study the effect of position. The p-BT and m-BT isomers were synthesized by the Suzuki cross-coupling reaction of BTD–TPE with boronate esters of BT. The Stille cross-coupling reaction was employed for o-BT. The structural features of the isomers endowed them with solvatochromism, mechanochromism, acidochromism and aggregation induced emission properties which were studied using emission and absorption spectroscopy. The reversible mechanochromic behaviour was associated with the phase transition from crystalline to amorphous and was used to develop rewritable ink free paper. The single crystal X-ray analysis of p-BT and o-BT establishes that mechanochromism synergistically depends on the flexibility and twisting in the donor and acceptor moieties. The isomers can sense trifluoroacetic acid in solution as well as the solid state. The chosen strategy allows modulation of fluorescence properties making them potential stimuli responsive materials with applications in mechano-sensors, security inks and optoelectronic-devices.


 Please wait while we load your content...
Please wait while we load your content...