Gated photochromism in azobenzene-appended rhodamine cassette: through-bond energy transfer – a universal strategy towards “Lock and Unlock” system†
Abstract
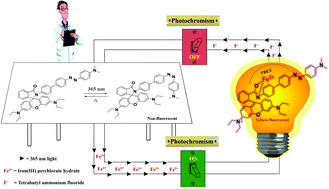
A dyad 1 comprising fluorescent rhodamine and photochromic azobenzene covalently linked through a phenylene bridge, which still retains their individual electronic properties, was synthesized. Gating between the fluorescent and photochromic properties of the dyad in the presence of external stimuli such as light or cations/anions or both paved the way for the development of new stimuli-responsive molecular materials. Among the several metal ions, Fe3+ ion was found to be selective in inducing the spirocyclic ring opening reaction of rhodamine, which can be witnessed through turn-on fluorescence. Fe3+-bound dyad 1 precluded the traditional trans → cis isomerization of remotely connected azobenzene and catalysed the spirocyclic ring opening reaction, which led to fluorescence enhancement by a factor of 640. The fluorescence enhancement originated from through-bond energy transfer upon excitation of azobenzene. Indeed, the turn-on fluorescence and optical changes upon UV irradiation indicated that this material can be used as a probe to monitor harmful UV radiation. The distinctly different responses of 1 concerning physical stimuli (light) and chemical stimuli (Fe3+ and F− ions) make this molecule a multi-addressable functional material.


 Please wait while we load your content...
Please wait while we load your content...