NIR polymers and phototransistors†
Abstract
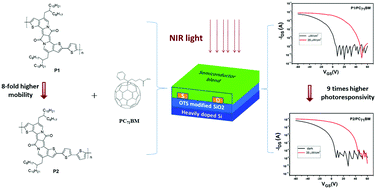
A novel bisthiophene-fused diketopyrrolopyrrole unit (4,11-bis(2-octyldodecyl)-7H,14H-thieno[3′,2′:7,8]indolizino[2,1-a]thieno[3,2-g]indolizine-7,14-dione, BTI) has been designed as an electron acceptor and used to copolymerize with thiophene and bithiophene as electron donors to construct two D–A conjugated polymers, P1 and P2via Stille coupling, respectively. The two polymers showed excellent thermal stability, broad light absorption and a narrow energy band gap. P1 and P2 were used to fabricate organic field-effect transistors (OFETs) to evaluate their charge transport characteristics. P2 showed much better hole transport performance with a mobility of 0.1 cm2 V−1 s−1. Near-infrared (NIR) phototransistors were also fabricated by using the two polymers blended with PC71BM as the active layer. With illumination of 35 μW cm−2 at a wavelength of 850 nm, the photocurrent/dark-current ratio (P) and photoresponsivity (R) of the phototransistor based on P1/PC71BM were 3.6 × 104 and 270 A W−1, respectively. For P2/PC71BM, P was 2.5 × 104 and R reached 2420 A W−1.


 Please wait while we load your content...
Please wait while we load your content...