Dual fluorescence switching of a Rhodamine 6G-naphthalimide conjugate with high contrast in the solid state†
Abstract
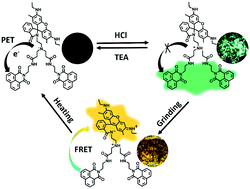
Solid-state fluorescence switches have attracted tremendous attention in recent years owing to their applications in fluorescent sensors, security inks and data storage. Herein, we report a novel molecule (2NR) composed of two naphthalimide moieties and a Rhodamine 6G (R6G) unit linked by a branched amidoamine spacer. 2NR exhibits excellent acid and force dual-stimuli-responsive behaviour in the solid state: (i) exposing 2NR powder to HCl vapour results in fluorescence off–on switching, because protonation of the tertiary amine by acid impedes the photoinduced electron transfer process; (ii) further treatment with grinding, the force-induced open-ring reaction of R6G and subsequent efficient fluorescence resonance energy transfer from naphthalimide to the open-ring form of R6G realizes a high-contrast fluorescent colour change. Moreover, the branched amidoamine spacer induces a twisted conformation of naphthalene moieties, which provides considerable free volume and significantly facilitates the isomerization process of R6G from spirolactam to open-ring amide. Both fluorescence switches have good reversibility. Consequently, the excellent solid-state fluorescent switching of 2NR is highly applicable for anti-counterfeiting materials.


 Please wait while we load your content...
Please wait while we load your content...