Anion-induced ionic liquid crystals of diphenylviologens†
Abstract
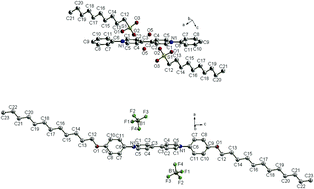
Two series of ionic liquid crystals derived from diphenylviologens, 1 and 2, were prepared and their photophysical and electrochemical properties were also investigated. The crystallographic data of two single crystals, 1-RSO3 (n = 10) and 2-BF4 (n = 12), showed that a mono- or bilayer-lamellar structure was induced by intermolecular H-bonds. All mesophases were identified as smectic A phases by POM textures and powder XRD data. A linear correlation plot (R2 = 0.8885) of the d-spacings vs. the calculated effective radius (Reff) in compounds 2-X was obtained. The aggregation-induced emission (AIE) ranging from ca. 463 to 529 nm observed in compounds 2-X (e.g., X = BF4, PF6, RSO3, OTf and NTf2) was also anion dependent. Also, compounds 2-X exhibited thermal switching, with an On/Off-emission mode between the Cr and SmA phases. In its cyclic voltammogram, compound 1-RSO3 (n = 12) exhibited two distinct reversible redox couples; in contrast, compound 2-RSO3 (n = 12) formed spike-like voltammetric peak potentials without redox couples.


 Please wait while we load your content...
Please wait while we load your content...