Opening of large band gaps in metallic carbon nanotubes by mannose-functionalized dendrimers: experiments and theory†
Abstract
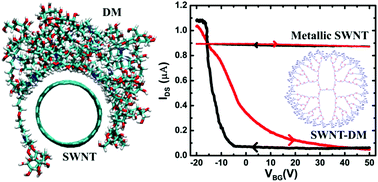
Despite many theoretical schemes, the direct experimental observation of supramolecular control over band gap opening in single-walled carbon nanotubes (SWNTs) is still lacking. We report an experimental and theoretical demonstration of a metal-to-semiconductor transition with a precisely measured large band gap in SWNTs due to the wrapping of mannose-functionalized poly(propyl ether imine) dendrimer (DM) molecules. The semiconductor behaviour of the SWNT–DM complex is comprehensively established with a band gap value of ∼0.45 eV measured using scanning tunnelling spectroscopy (STS), ionic liquid top-gated field-effect transistor (FET) characteristics and Raman spectroscopy. Further, a validated molecular picture of the SWNT–DM complex obtained from fully atomistic molecular dynamics (MD) simulations was used to carry out ab initio density functional theory (DFT) and GW calculations of the electronic structure, evaluating an experimentally estimated band gap value. We attribute this large band gap opening in SWNTs to the complexation-induced asymmetric strain developed in the carbon–carbon bond length.


 Please wait while we load your content...
Please wait while we load your content...