Coordination of the chemical and electronic processes in GaSb(100) surface modification with aqueous sodium sulfide solution
Abstract
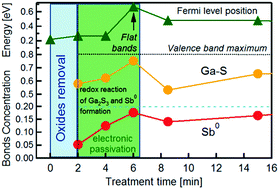
The mechanism of the chemical and electronic passivation of the GaSb(100) surface with aqueous sodium sulfide solution is investigated by X-ray photoemission spectroscopy and via the monitoring of the solution pH value over the course of the surface exposure to the solution. Upon the exposure of the native-oxide-covered GaSb(100) surface to 1 M aqueous Na2S solution, the solution pH remains constant for 6 min, then increases suddenly for 1 min, and afterwards, it decreases steeply. During the first two minutes of exposure of the native-oxide-covered GaSb(100) surface to the solution, the amount of surface oxide drastically decreases and the surface becomes antimony-rich, whereas the GaSb surface electronic structure is affected only slightly. Longer exposure of GaSb(100) to the solution causes the accumulation of gallium sulfides and the oxidation of the semiconductor surface antimony atoms to elemental antimony. The extra electrons released due to such an oxidation reduce the protons split from the water molecules at the semiconductor/solution interface. These chemical processes are accompanied by the essential modification of the surface electronic properties, indicating the importance of the redox processes in the modification of the GaSb(100) surface electronic structure.


 Please wait while we load your content...
Please wait while we load your content...