Proton conductive cationic nanoporous polymers based on smectic liquid crystal hydrogen-bonded heterodimers†
Abstract
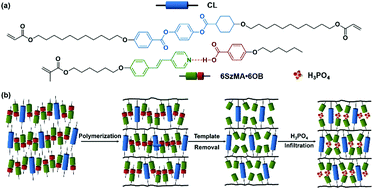
The fabrication of a cationic nanoporous smectic liquid crystal network (LCN) based on hydrogen bonded heterodimers is presented. The method relies on a supramolecular complex made from a pyridyl bearing reactive mesogen hydrogen bonded to a non-reactive benzoic acid template. Upon addition of a cross-linker, a smectic liquid crystalline phase is obtained that can be fixed by photopolymerization. It was found that the lamellar structure was maintained after template removal when 25 wt% or more cross-linker was used, yielding a nanoporous LCN. After H3PO4 immobilization in the pores of the LCN, a cationic 2D nanoporous polymer is obtained showing high and anisotropic anhydrous proton conductivity.


 Please wait while we load your content...
Please wait while we load your content...