Proton-conductive materials formed by coumarin photocrosslinked ionic liquid crystal dendrimers†
Abstract
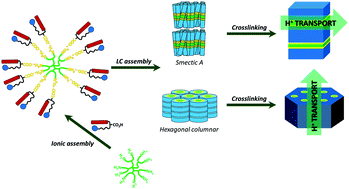
In this work, we have successfully examined for the first time the use of ionic dendrimers as building blocks for the preparation of 1D and 2D proton conductive materials. For this purpose, a new family of liquid crystalline dendrimers has been synthesized by ionic self-assembly of poly(amidoamine) (PAMAM) dendrimers bearing 4, 8, 16, 32 or 64 NH2 terminal groups and a coumarin-containing bifunctional dendron. The noncovalent architectures were obtained by the formation of ionic salts between the carboxylic acid group of the dendron and the terminal amine groups of the PAMAM dendrimer. The liquid crystal properties have been investigated by polarized optical microscopy (POM), differential scanning calorimetry (DSC) and X-ray diffraction (XRD). All the compounds exhibited mesogenic behavior with smectic A or hexagonal columnar mesophases depending on the generation of the dendrimer. Coumarin photodimerization was used as a crosslinking reaction to obtain liquid crystalline polymer networks. All the materials showed good proton conductive properties as the LC arrangement leads to the presence of ionic nanosegregated areas (formed by the ion pairs) that favor proton conduction.


 Please wait while we load your content...
Please wait while we load your content...